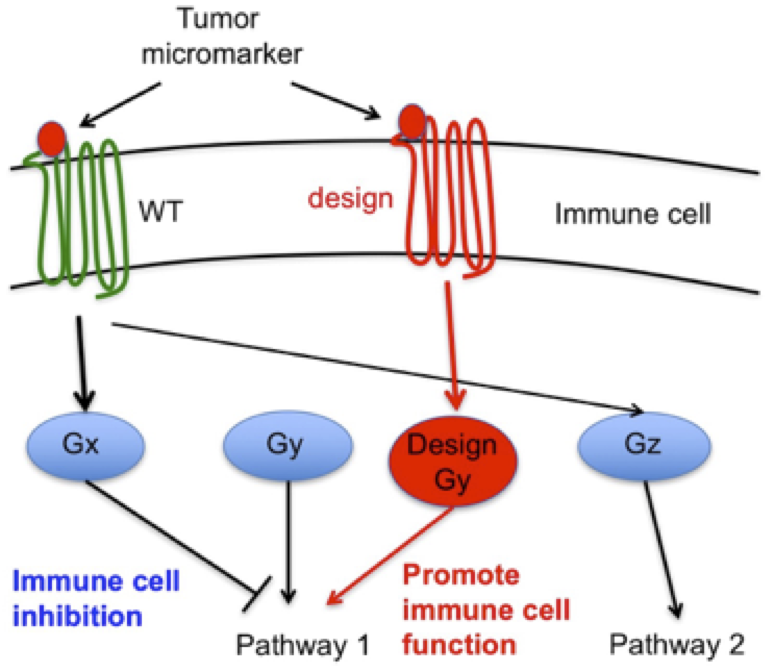
Immunotherapy has the potential to significantly impact treatment outcomes of cancer patients. Adoptive T cell therapies with genetically engineered T cells for example have produced impressive responses in leukemia patients, but to achieve similar outcomes in other cancers, additional strategies are likely required. The tumor microenvironment is complex and contains many immunosuppressive factors that can contribute to failure of adoptively transferred T cells. Therefore, we strive to design protein systems that redirect immune-inhibitory tumor microenvironment stimuli into an activating/proliferative response in tumor-specific T cells through engineered receptors. This strategy involves the creation of receptor chimera that couple extracellular stimuli to unrelated intracellular responses. However, such approaches are currently limited by the lack of understanding of molecular determinants of receptor signaling. We address these limitations through two novel approaches in collaboration with experts in immunotherapy at the Ludwig Cancer Center in Lausanne (e.g. Dr. Caroline Arber, Dr. George Coukos) to select, validate in vivo and support the translation of designed receptors from bench to clinical application in T cell therapies.
First, we combine our modeling, design techniques with experimental screening and selection approaches to identify novel receptors (Arber et al., J Clin Inv 2015) and assess how domains from distinct receptors can be combined and reengineered to create receptor chimera eliciting intended signals. Second, instead of redesigning intrareceptor signaling properties, extracellular signals can be redirected by designing novel signaling partners. For that purpose, in proof of concept studies (Young et al., under review), we have recently designed novel orthogonal GPCR/G protein molecular switches where designed receptors only bind and signal through designed effector G proteins without interference from native receptors or effectors. Additionally, we engineer these systems such that designed receptors can trigger non-native signaling pathways, thereby providing molecular signaling switches that can be “plugged” in native cellular context to redirect signals (Arber et al., COB 2017). In principle, this strategy could be applied to redirect GPCR systems in any cell type used in immunotherapy.