Research aim
To cope with fluctuating nutrient supply and other dynamic changes, growing tissues in development and cancer rapidly adapt their gene expression through post-transcriptional regulation of mRNA translation and protein turnover. We investigate how these processes are regulated in tubular epithelial cells by K-homology domains and a Sterile Alpha Motif (SAM) of the RNA-binding protein Bicaudal-C (BICC1) and by specific interacting factors associated with impaired cilia signaling during development and in cystic kidney diseases.How does BICC1 interact with factors implicated in polycystic kidney diseases and other ciliopathies?
Besides randomizing the visceral situs, loss of Bicc1 provokes pancreatic and bile duct dilatation and renal cysts reminiscent of polycystic kidney diseases. Similar combinations of malformation occur in a number of syndromes linked to defects in primary cilia. Polycystic kidneys also share several cancer hallmarks, including sustained proliferation signals and alterations in glucose and lipid metabolism. How the regulation of these pathways is coupled to cilia is an area of intense investigation.
Loss of Bicc1 function provokes renal cysts
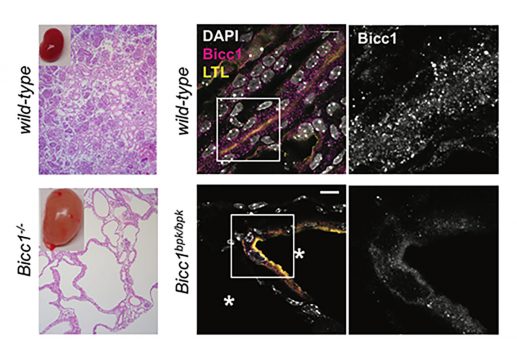
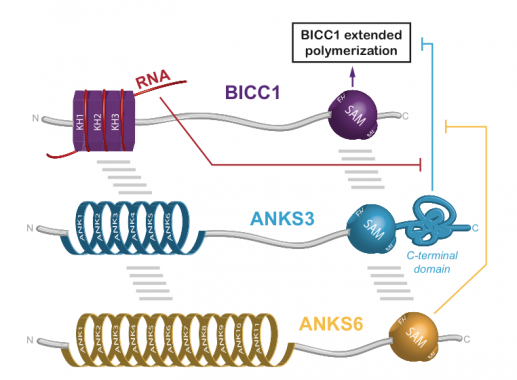
We found that Bicc1 coalesces in cytoplasmic aggregates through the sterile alpha motif (SAM). A mutation in the SAM domain that provokes renal cysts in Bicc1bpk/bpk mutant mice disperses these granules. SAM domain self-interactions are also blocked by the bpk mutation in GST pull-down assays. As a control, we specifically mutated the SAM-SAM interface (mutD). 3D modelling predicted that helical polymers with 6 SAM domains per turn position their N-termini at the periphery. The RNA-binding KH domains of Bicc1 thus are likely arrayed around a central helix formed by SAM domain polymers.
Rothé et al., 2015
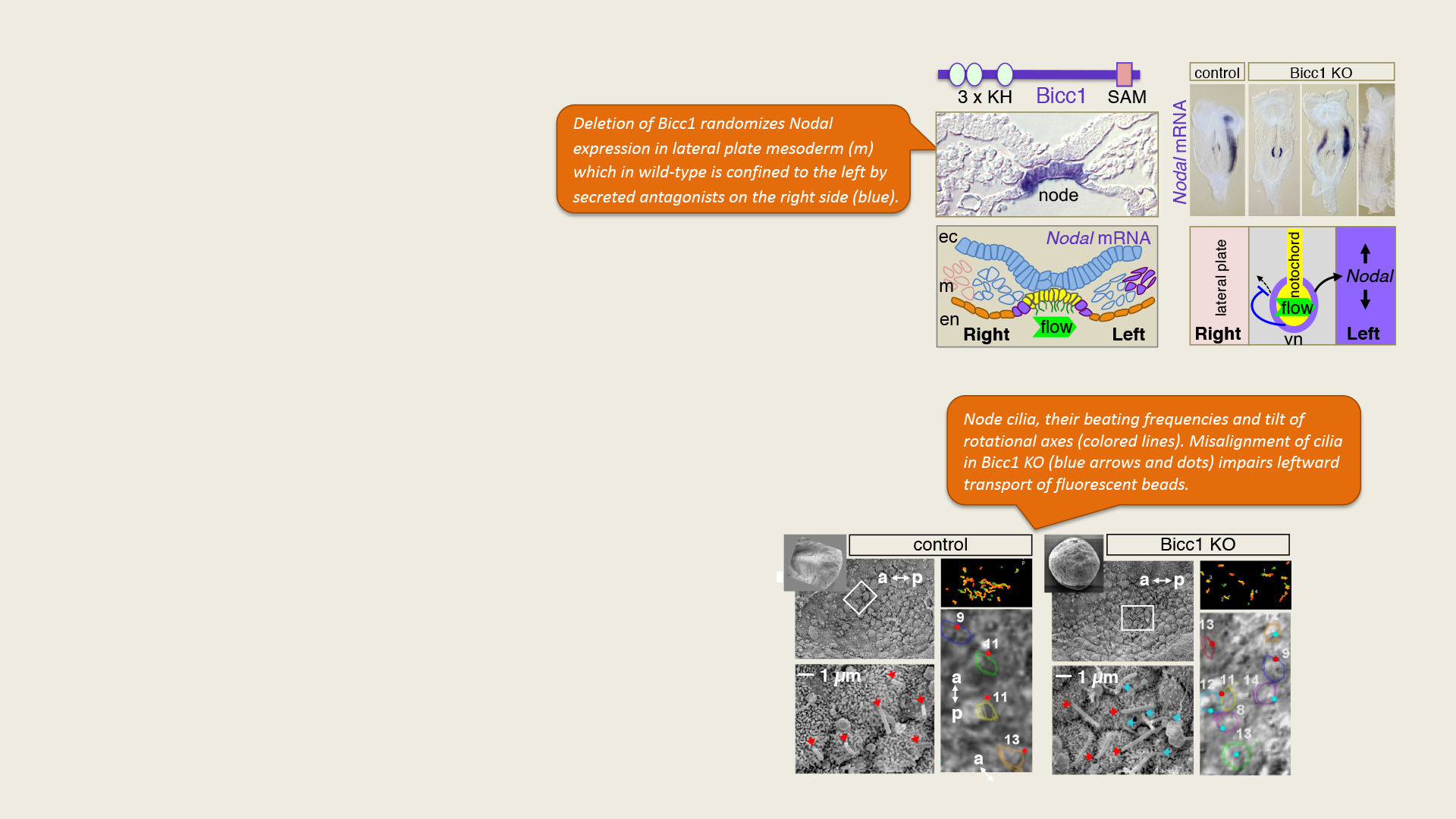
Fluid-filled cysts develop both in Bicc1-/- and in Bicc1 bpk/bpk mutant kidneys (asterisks). Immunostaining labels cytoplasmic Bicc1 foci in the wild-type that become diffuse in cyst-lining bpk/bpk mutant kidneys (magenta). In the merge, proximal tubule cells are stained yellow, nuclei in white. A zoom of the boxed area shows Bicc1 staining in white.
Bicc1 self-polymerization
3D modelling of Bicc1 polymers. Residues D913, K915 and E916 that were mutated to alanines to disrupt the SAM-SAM interface are highlighted. A diagram of full-length Bicc1 with polymerized SAM domains at the center and KH domains arrayed at the periphery is shown to the right.
Bicc1 polymers repress target mRNA translation
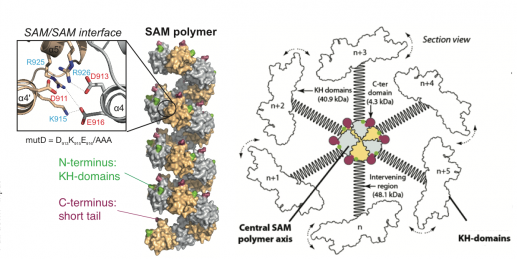
Primary cilia harbor G-protein coupled receptors that locally stimulate cAMP synthesis by adenylate cyclases. Calcium signals mediated by polycystin-2 counteract the resulting growth-promoting signals. How are these opposing ciliary signaling inputs balanced? Bicc1 curbs cAMP production by attenuating the translation of AC6 mRNA. Interestingly, a luciferase reporter containing a 3’UTR fragment of AC6 mRNA is recruited to cytoplasmic Bicc1 polymers, leading to translational repression. By contrast, mRNA binding to diffusely distributed polymerization mutant Bicc1 does not repress translation. How Bicc1 self-polymerization promotes mRNA silencing, and how this process is regulated by ciliopathy-associated mutations in specific interacting factors such as ANKS3 and ANKS6 remains to be determined.
Bicc1 polymers localize bound reporter mRNA
3’UTR reporter mRNA with 24 hairpins (LucAC6-MS2) that retain nuclear YFP-tagged MS2 coat protein in the cytoplasm is recruited to Bicc1 granules or to the diffusely distributed polymerization mutant mutD, respectively.
Rothé et al., 2018
Regulation of Bicc1 polymerization by the ciliopathy proteins ANKS3 and ANKS:
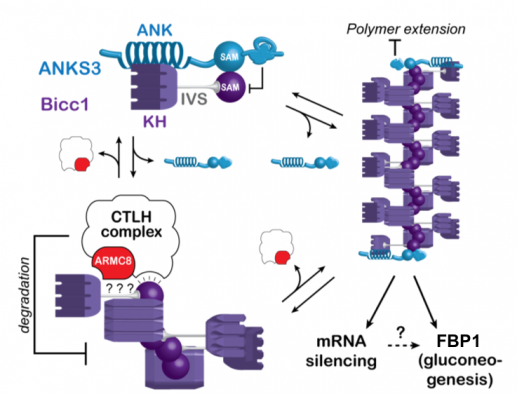
A screen for interacting factors revealed that Bicc1 polymers can be capped and rearranged by heterooligomers of the ankyrin repeat- and SAM domain-containing proteins (ANKS) 3 and 6.
A new role for Bicc1 in renal gluconeogenesis
Besides ANKS3 and ANKS6, TAP-tagged Bicc1 co-purified the so-called CTLH complex. The orthologous complex in S. cerivisiae promotes glycolysis by targeting gluconeogenic enzymes for proteasomal degradation. We mapped the CTLH interactions with Bicc1 and tested potential effects on cell metabolism. Loss of Bicc1 in newborn mice provoked hypoglycemia and depleted gluconeogenic enzymes in kidneys but not in the liver. However, rather than directly regulating gluconeogenic enzymes, mammalian CTLH complex targets Bicc1, competing for the same SAM domain interface that is also required for Bicc1 polymerization (Leal-Esteban et al., 2018). To what extent these novel SAM interaction partners regulate known Bicc1 functions in mRNA silencing, or whether they primarily act as specific downstream effectors remains to be determined.
Leal-Esteban et al., 2018
The same SAM domain surfaces that mediate Bicc1 self-polymerization or capping by ANKS3 alternatively are targeted by the CTLH complex to limit Bicc1 accumulation. How Bicc1 stimulates the expression of fructose-1,6-biphosphatase 1 (FBP1) and several other gluconeogenic enzymes is unknown.
Publications related to this project:
L. Leal-Esteban; B. Rothe; S. Fortier; M. Isenschmid; D. Constam : Role of Bicaudal C1 in renal gluconeogenesis and its novel interaction with the CTLH complex; PLoS Genetics. 2018. DOI : 10.1371/journal.pgen.1007487.
B. Rothe; C. Leettola; L. Leal-Esteban; D. Cascio; S. Fortier et al. : Crystal structure of Bicc1 SAM polymer and mapping of interactions between the ciliopathy-associated proteins Bicc1, ANKS3, and ANKS6; Structure. 2018. DOI : 10.1016/j.str.2017.12.002.
B. Rothe; L. Leal-Esteban; F. Bernet; S. Urfer; N. Doerr et al. : Bicc1 polymerization regulates the localization and silencing of bound mRNA; Molecular and Cellular Biology. 2015. DOI : 10.1128/Mcb.00341-15.
C. Maisonneuve; I. Guilleret; P. Vick; T. Weber; P. Andre et al. : Bicaudal C, a novel regulator of Dvl signaling abutting RNA-processing bodies, controls cilia orientation and leftward flow; Development. 2009. DOI : 10.1242/dev.038174.