*****
Did you know that, in many cultures, candles are considered to create a link between our world and the mystical universe? It often represents life or the human soul when lit. It is an aesthetic element that favors contemplation, closeness, protection, or poetry. Often ideal to close a door and open new possibilities. If you are not that otherworldly, and still want to light a candle to create a cozy, sweet-smelling atmosphere, let’s go for it!

Step 1: Get a candle.
Candles can be found in any supermarket today, and they are various in shape, color, smell, and composition. A candle is mainly made of two parts, a wax-body, and a wick.
The wax body is a mix of wax, that is generally paraffine wax, but can also be beeswax or plant-based wax (e.g. soybean, palm), and dyes, additives and perfumes. More specifically, paraffine is a chemically bleached and deodorized petroleum waste.
As for the wick, many candles have cotton core wicks wrapped around a metal holder. This design helps prevent the wick from falling into the wax. The metal holder could be lead, zinc or tin. On the other hand, some wicks are made of paper-core, cotton, or wood.
Step 2: Lit it.
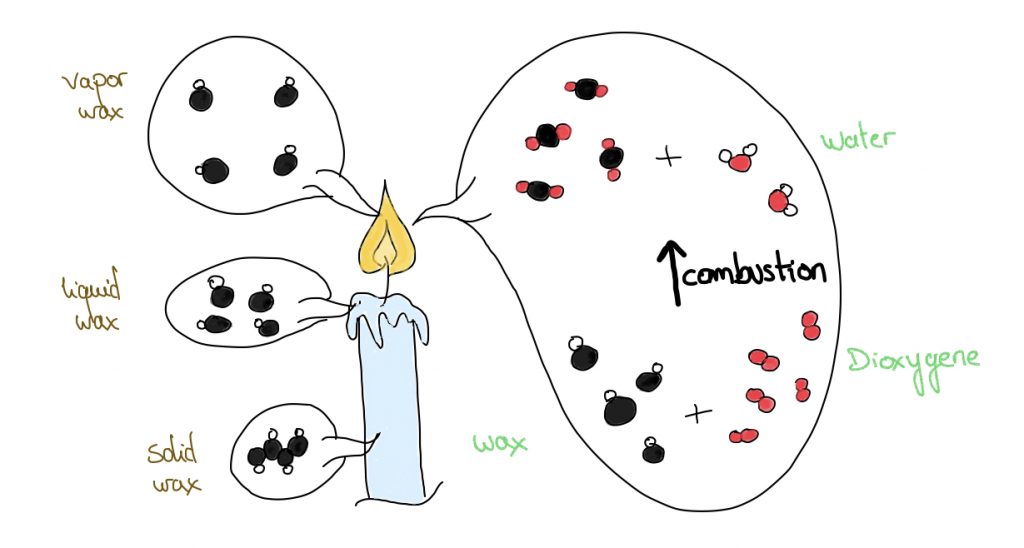
Lighting a candle is based on the physical phenomenon of combustion. The flame is the result of the combustion of the molecules which constitute the wax. Indeed, when a candle is lit, the heat from the flame melts the wax, which vaporizes and enters a combustion process where the hydrocarbons interact with oxygen. From this reaction comes mainly heat, light, carbon dioxide and water.
When paraffine burns, volatile organic compounds (VOCs) are released into the air [1]. These VOCs include benzene and toluene, but also alkenes and acetone. The benzene is a gas emitted by forest fires, volcanoes and burning coal or oil. But 90% of its exposure occurs as a consequence of smoking [2]. As for toluene, it is a gas widely used in paint thinners and adhesives.
In addition to the organic gases released, the combustion of paraffine produces soot composed of particles such as PM2.5 (fine particles, less than 2.5 micrometers in diameter) and UFP (ultra-fine particles, less than 100 nanometers in diameter) [3]. These particles can remain in suspension for hours.
As for chemical perfumes and synthetic dyes, their combustion results in the formation of formaldehyde, petroleum distillate, limonene, alcohol and ester.
Finally, all metal core wicks release traces of heavy metals into the air when burned [4]. It should be noted that zinc and tin core wicks may also release small amounts of lead particles. Measurements of the amount of lead emitted from the combustion of 14 different brands of candles (Michigan, USA) with metal wicks resulted in the following results: 0.5 to 66 μg/h for lead wicks, and 1.2 to 124 μg/h for zinc wicks.
Step 3: Enjoy it. But any symptoms?
That sweet smell of vanilla and that flickering flame got you, didn’t it? And as a bonus, invisible fumes of fancy names like toluene and formaldehyde embalmed the room. But unfortunately, behind that atmosphere are toxic compounds that could damage human health.
Here, as a first step, it is a matter of looking at the intrinsic health risks of the products emitted by paraffine candles. It must be recognized that the question of whether the concentration emitted of these products endanger human health is a controversial issue. This will be developed further in the following.
The above-mentioned VOCs are known carcinogens [5]. Exposure to benzene, in particular, increases the risk of leukaemia and other blood cancers. It should also be mentioned here that the VOCs produced can react with ambient ozone (e.g. released by an ion generator) and produce a range of oxidation by-products, including formaldehyde and UFPs, which are potentially toxic molecules [6].
As for the ultrafine particles, they penetrate deep into the lungs and are absorbed into the bloodstream. They are often associated with serious health problems such as COPD (chronic obstructive pulmonary disease), respiratory tract infections, or stroke [7].
If some components can cause long-term health problems, others make their effects felt on the spot. For example, benzene can cause allergies, asthma attacks and skin problems. Toluene or formaldehyde can also lead to eye, nose, throat and skin irritation, headaches, dizziness or confusion [2].
As discussed earlier, these harmful compounds usually emanate from the burning of the candle. However, according to a study published in 2005 by the University of South Florida, paraffine wax candles emit low levels of benzene even when unlit and at room temperature [8].
As for lead wicks, inhalation of particles from the combustion of this metal is particularly harmful to young children. For a given source, they absorb 4 to 5 times more lead per ingested amount than adults. This can have serious and permanent consequences on their health, particularly on the development of the brain and nervous system. Lead also has long-term deleterious effects in adults, including increased risk of high blood pressure and kidney damage. Also, exposure of pregnant women to high levels of lead can result in miscarriage, stillbirth, premature birth and low birth weight [9].
Step 4: Keep it or throw it?
In toxicology, it is usually said that the dose makes the poison. The sole question of which compounds are emitted is therefore not sufficient. The size of the room, the number of candles lit, the duration for which they are lit, or whether the room is ventilated are all parameters to be considered to determine the nature of the exposure [10].
First of all, the statement about metal wick candles is pretty clear among the scientific community. It has been estimated that burning a metal wick candle (Michigan, USA) for 2 hours can result in airborne lead concentrations that may be harmful to human health [4]. Knowing that other types of wicks emit little or no lead when burned (e.g. paper wicks, cotton), it is clearly possible to avoid them in view of the health threats they may represent.
Secondly, while the study of the University of Southern California advises not to use paraffine candles for the nature of their emissions, another study published in the Journal of Regulatory Toxicology and Pharmacology counters [11]. The latter consisted in evaluating the contribution of scented candles to indoor load of airborne substances and particulate matter in a controlled environment [12]. Health risks to consumers were characterized by comparing exposure concentrations to ambient air quality guidelines or to established toxicity thresholds. The results were that the highest emission values of fragrances, formaldehyde and benzene were all below the air quality exposure limits defined by the World Health Organization [13]. Thus, according to this study, scented paraffin candles do not pose any health risks when used under normal conditions (we must admit that “normal” is relative…). Moreover, an other study found that even when many candles would be burnt at the same time in a small room, concentrations of the compounds investigated (VOCs and Polycyclic aromatic hydrocarbons) stay 1 % of the tolerable limit values [14].
However, to get away from this scientific disagreement, it is possible to think of alternatives. In fact, a 2002 study published in the Journal of the American Oil Chemists’ Society found that soy wax, like beeswax candles, burn at a significantly lower rate and produce less soot than paraffin candles [15].
But in reality, inhaling large amounts of smoke from any type of wax is harmful to health.
So we come to the conclusion that all this does not mean that we should throw our candles in the bin, but rather that we should use them in a reasoned way. Some precautionary advice would be to use them modestly, avoiding inhaling directly the smoke and ensuring adequate ventilation of the room [16].
As Dr. Croft, assistant professor of pulmonary and critical care medicine at the University of Rochester Medical Center, so aptly summarizes: “Studies are actively underway regarding the emissions from candles and trying to answer these important questions. I don’t want anyone to think ‘Okay, well it’s settled, candles are safe.’ This is an active area of research and it’s an area that we all need to follow and while all the research develops, moderation is really key.” [17].
Step 5: Meditate…
A truly pure air does not smell anything.
Acknowledgment
Special thanks to Professor Dusan Licina (EPFL) for the knowledge provided in the Indoor Air Quality and Ventilation field.
References
[1] Derudi, M., Gelosa, S., Sliepcevich, A., Cattaneo, A., Rota, R., Cavallo, D. M., & Nano, G. (2012). Emissions of air pollutants from scented candles burning in a test chamber. Atmospheric Environment, 55, 257–262. https://doi.org/10.1016/j.atmosenv.2012.03.027
[2] Milnea. (2022, December 19). Worried if Candles Are Toxic? Cleveland Clinic. https://health.clevelandclinic.org/are-candles-bad-for-you/
[3] Fine, P. R., Cass, G. R., & Simoneit, B. R. (1999). Characterization of Fine Particle Emissions from Burning Church Candles. Environmental Science & Technology, 33(14), 2352–2362. https://doi.org/10.1021/es981039v
[4] Nriagu, J. O., & Kim, M. (2000). Emissions of lead and zinc from candles with metal-core wicks. Science of the Total Environment, 250(1–3), 37–41. https://doi.org/10.1016/s0048-9697(00)00359-4
[5] Rumchev, K., Brown, H., & Spickett, J. (2007). Volatile Organic Compounds: Do they present a risk to our health? Reviews on Environmental Health, 22(1). https://doi.org/10.1515/reveh.2007.22.1.39
[6] Stevanovic, S. (n.d.). Too many smelly candles? Here’s how scents impact the air quality in your home. The Conversation. https://theconversation.com/too-many-smelly-candles-heres-how-scents-impact-the-air-quality-in-your-home-190913
[7] Schraufnagel, D. E. (2020). The health effects of ultrafine particles. Experimental and Molecular Medicine, 52(3), 311–317. https://doi.org/10.1038/s12276-020-0403-3
[8] Silver, D. J. (2005). Occupational Exposure to Ultrafine Particles and Polycyclic Aromatic Hydrocarbons from Candle Emissions.
[9] World Health Organization: WHO. (2022b). Lead poisoning. www.who.int. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health
[10] Maupetit, F., & Squinazi, F. (2009). Caractérisation des émissions de benzène et de formaldéhyde lors de la combustion d’encens et de bougies d’intérieur : élaboration de scénarios d’exposition et conseils d’utilisation. Environnement Risques & Sante, 8(2), 109–118. https://doi.org/10.1684/ers.2009.0235
[11] Candles: What do they emit when lit? (n.d.). Office for Science and Society. https://www.mcgill.ca/oss/article/student-contributors-you-asked-general-science/candles-what-do-they-emit-when-lit
[12] Petry, T. M., Vitale, D., Joachim, F. J., Smith, B. J., Cruse, L. W., Mascarenhas, R., Schneider, S., & Singal, M. (2014). Human health risk evaluation of selected VOC, SVOC and particulate emissions from scented candles. Regulatory Toxicology and Pharmacology, 69(1), 55–70. https://doi.org/10.1016/j.yrtph.2014.02.010
[13] WHO Guidelines for Indoor Air Quality: Selected Pollutants. (2010). World Health Organization.
[14] Lau, C. S., Fiedler, H., Hutzinger, O., Schwind, K. H., & Hosseinpour, J. (1997). Levels of selected organic compounds in materials for candle production and human exposure to candle emissions. Chemosphere, 34(5–7), 1623–1630. https://doi.org/10.1016/s0045-6535(97)00458-x
[15] Rezaei, K., Wang, T., & Johnson, L. A. (2002). Combustion characteristics of candles made from hydrogenated soybean oil. Journal of the American Oil Chemists’ Society, 79(8), 803–808. https://doi.org/10.1007/s11746-002-0562-y
[16] Orecchio, S. (2011). Polycyclic aromatic hydrocarbons (PAHs) in indoor emission from decorative candles. Atmospheric Environment, 45(10), 1888–1895. https://doi.org/10.1016/j.atmosenv.2010.12.024
[17] Phoenix, K. (2022, December 25). Are Candles Bad for Your Health? Experts Explain. Good Housekeeping. https://www.goodhousekeeping.com/health/a42318715/are-candles-bad-for-you/
Featured image: https://cdn.unitycms.io/images/8tCb82Zm4JRAK-mrQfH0HS.jpg?op=ocroped&val=2001,2000,1000,1000,0,0&sum=rCiJ9JRzvHQ
Figure 1: https://blog.polarshade.com/hubfs/bigstock-Closeup-of-burning-candles-spr-247358983.jpg
Figure 2: S. Jouhari, 2023
Figure 3: https://streamline.imgix.net/84559ca3-22b1-4190-8518-19c1e2c6e670/784e0a2e-2029-4e33-aee9-dbee25510934/Know%20the%20Difference.png?ixlib=rb-1.1.0&w=2000&h=2000&fit=max&or=0&s=0426c6a272b26b230f4edca0260e9978