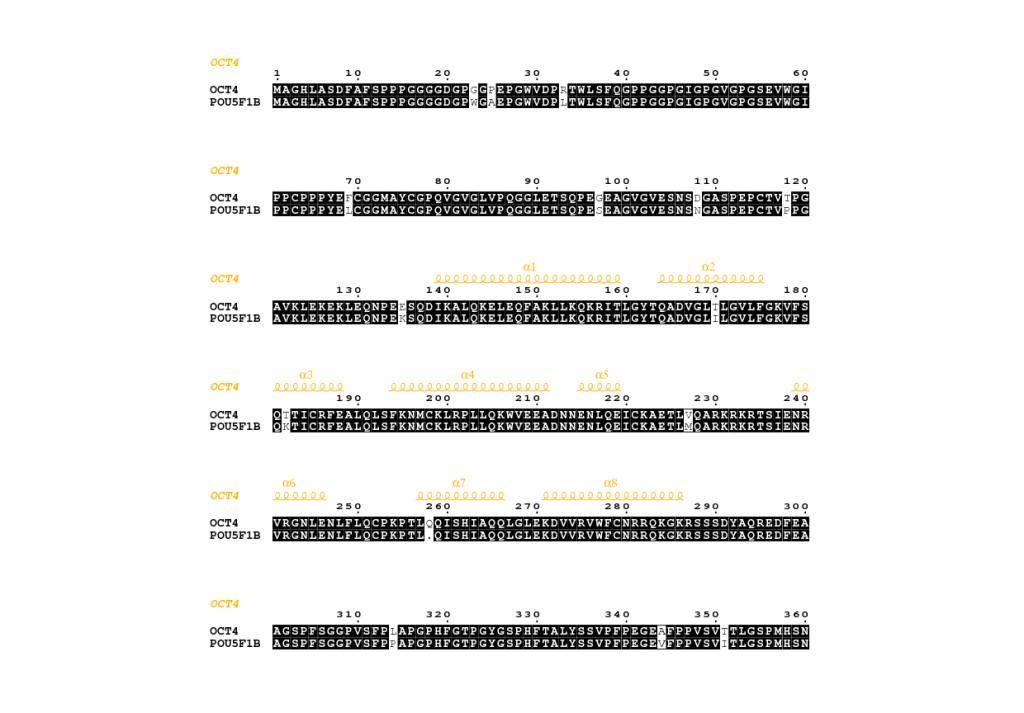
Investigating the critical amino acids differentiating POU5F1B from its parent OCT4
OCT4 is a member of the POU-domain transcription factor family, proteins that regulate the expression of their target genes by binding to an octameric DNA motif. Hitherto, seven OCT4-related pseudogenes have been identified, sharing high homology with their parent gene. One of them, POU5F1B, encoding a protein 95% homologous with OCT4 is transcribed in cancer cells. Despite the affiliation of POU5F1B with numerous tumors, its biophysical behavior has not been investigated. Hence, we employ a synergetic approach to study POU5F1B at a molecular and structural level in collaboration with Dr. L. Simo in the Trono lab at EPFL. Our results show that despite their high sequence similarity, POU5F1B and OCT4 have different subcellular localization and biophysical behavior. To tackle this experimentally we introduce mutations in OCT4 that resemble POU5F1B and vice versa, trying to decipher which amino acids are responsible for the difference in protein solubility and localization.