Current projects:
1. Developing methods to study dynamics of chromatin regulation
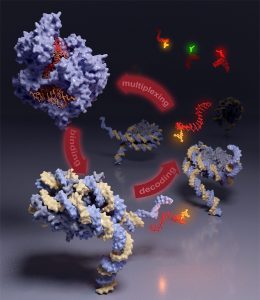
The structure of chromatin is organized over many length scales and highly dynamical. Its function is modulated by the combinatorial effect of histone post-translational modifications (PTMs). Specialized reader proteins interpret combinations of PTMs and result in structural transition in chromatin, recruitment of cellular machinery and gene regulation.
We develop chemical methods to site specifically introduce PTMs and fluorescent labels into reconstituted synthetic chromatin fibers to study conformational dynamics in chromatin as a function of histone marks (e.g. ubiquitylation) and structural reader proteins on the single molecule scale.
We are interested in how proteins readers find their site of action in the highly complex environment of the cell. Thus, we characterize the binding thermodynamics and binding kinetics of protein readers with modified chromatin in vitro and study their diffusional properties and localization in cells.
Relevant publications:
Multivalency governs HP1α association dynamics with the silent chromatin state, 18 Jun 2015 | doi:10.1038/ncomms8313
https://www.nature.com/nsmb/journal/vaop/ncurrent/full/nsmb.3488.html
http://www.nature.com/nrg/journal/vaop/ncurrent/abs/nrg.2017.28.html
https://pubs.acs.org/doi/10.1021/jacs.1c06195
2. Deciphering the molecular basis of gene activation
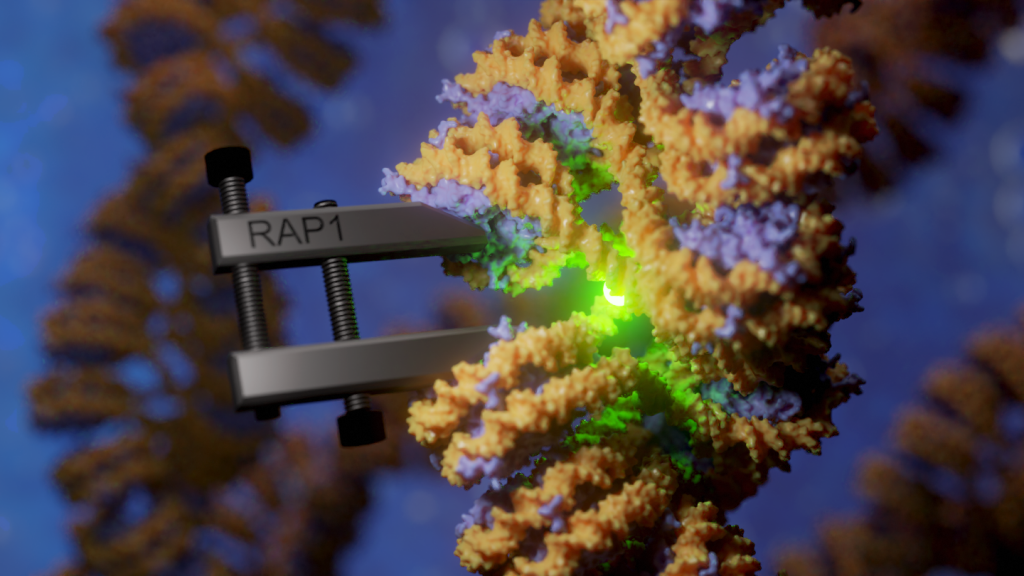
How doe transcription factors, chromatin remodelers and the RNA polymerase collaborate to activate genes? Here, we use a combination between chemical biology, single-molecule biophysics and cell imaging to unravel dynamic processes in gene control with molecular details.
Relevant publication:
https://www.cell.com/molecular-cell/pdfExtended/S1097-2765(19)30803-2
3. Chemical biology of chromatin and the cytoskeleton
 |
The study of the function of post-translational modifications requires access to chemically defined samples. Thus, we develop and apply novel chemical methods to site-specifically modify chromatin proteins, e.g. controlling the ubiquitylation state, and cytoskeletal proteins such as tubulin.
Relevant publications:
Traceless synthesis of asymetrically modified bivalent nucleosomes
https://pubs.rsc.org/en/content/articlelanding/2017/cc/c7cc06180c
http://pubs.rsc.org/en/content/articlehtml/2017/cc/c7cc06180c