Publications
2025
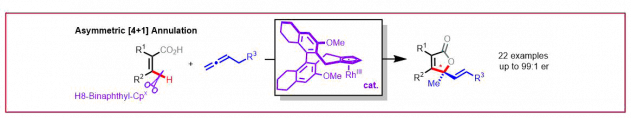
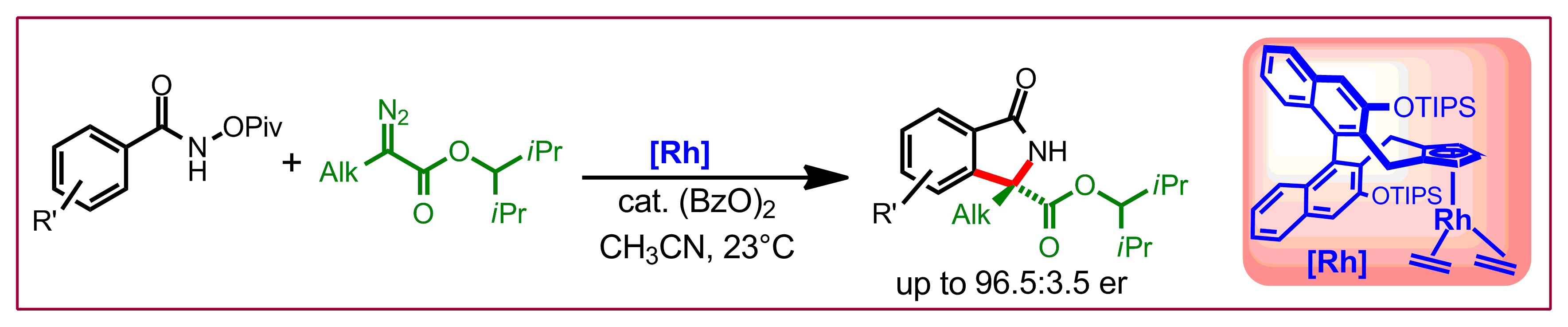
L. K. Verdhi, M. D. Wodrich, N. Cramer, J. Am. Chem. Soc. 2025, XXX, XXXXX-XXXXX: “Enantioselective Cobalt(III)-Catalyzed [4 + 1] Annulation of Benzamides: Cyclopropenes as One-Carbon Synthons”
S. Jana, M. D. Wodrich, N. Cramer, Nat. Commun. 2025, 16, 3293-3301: “Enantioselective acyl-trifluoromethylation of olefins by bulky thiazolium carbene catalysis”
2024
S. Jana, N. Cramer, J. Am. Chem. Soc. 2024, 146, 35199-35207: “Tunable Thiazolium Carbenes for Enantioselective Radical Three-Component Dicarbofunctionalizations“
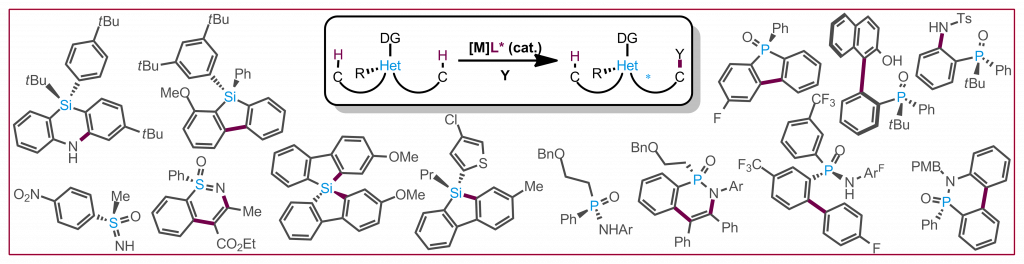
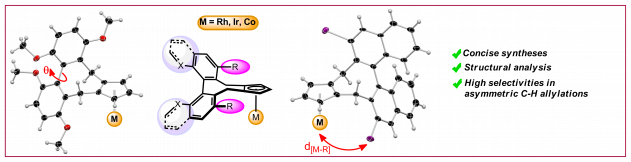
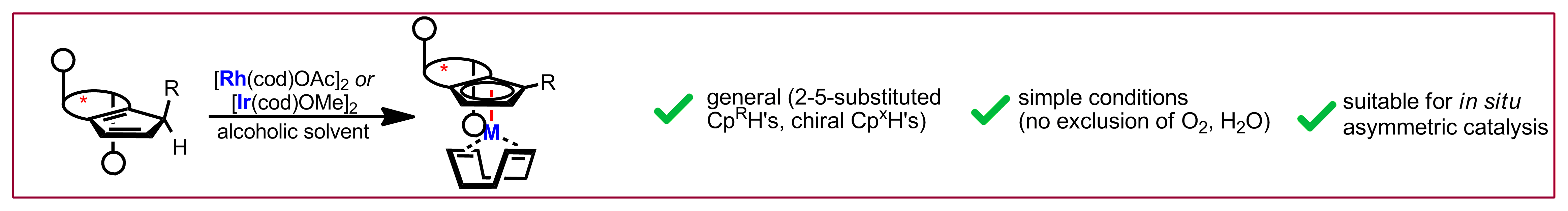
Y. S. Ye, A. Laverny, M. D. Wodrich, R. Laplaza, F. Fadaei-Tirani, R. Scopelliti, C. Corminboeuf, N. Cramer, J. Am. Chem. Soc. 2024, 146, 34786-34795: “Enantiospecific Synthesis of Planar Chiral Rhodium and Iridium Cyclopentadienyl Complexes: Enabling Streamlined and Computer-Guided Access to Highly Selective Catalysts for Asymmetric C–H Functionalizations“
Y.-X. Cao, A.-S. Chauvin, S. Tong, L. Alama, N. Cramer, ChemRxiv 2024, doi:10.26434/chemrxiv-2024-3w4p4: “Accessing Carbon, Boron and Germanium Spiro Stereocenters in a Unified Catalytic Enantioselective Approach“
A. Madron du Vigné, N. Cramer, Chem. Sci. 2024, 15, 13864-13871: “Streamlined synthetic assembly of α-chiral CAAC ligands and catalytic performance of their copper and ruthenium complexes“
Y. Zhang, J.-J. Zhang, L. Lou, R. Lin, N. Cramer, S.-G. Wang and Z. Chen, Chem. Soc. Rev. 2024, 53, 3457-3484: “Recent advances in Rh(I)-catalyzed enantioselective C–H functionalization“
G. Zhang, M. D. Wodrich, N. Cramer, Science 2024, 383, 395-401: “Catalytic enantioselective reductive Eschenmoser-Claisen rearrangements“
2023
P. Laveille, P. Miéville, S. Chatterjee, E. Clerc, J.-C. Cousty, F. de Nanteuil, E. Lam, E. Mariano, A. Ramirez, U. Randrianarisoa, K. Villat, C. Copéret, N. Cramer, Chimia 2023, 77, 154-158: “Swiss Cat+, a Data-driven Infrastructure for Accelerated Catalysts Discovery and Optimization”
M. D. Wodrich, R. Laplaza, N. Cramer, M. Reiher, C. Corminboeuf, Chimia 2023, 77, 139-143: “Toward in silico Catalyst Optimization”
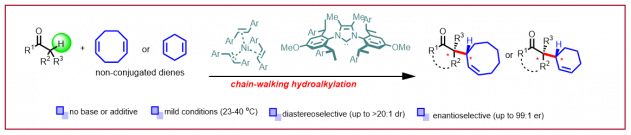
Y.-X. Cao, M. D. Wodrich, N. Cramer, Nat. Commun. 2023, 14, 7640: “Nickel-catalyzed direct stereoselective α-allylation of ketones with non-conjugated dienes“
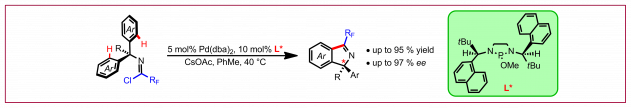
G. Zhang, N. Cramer, Angew. Chem. Int. Ed. 2023, e202301076: “Reductive Asymmetric Aza-Mislow-Evans Rearrangement by 1,3,2-Diazaphospholene Catalysis”
2022
A. Madron du Vigné, N. Cramer, Organometallics 2022, 41, 2731–2741: “Chiral Cyclic Alkyl Amino Carbene (CAAC) Transition-Metal Complexes: Synthesis, Structural Analysis, and Evaluation in Asymmetric Catalysis”
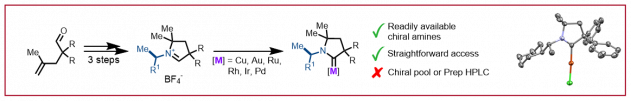
O. Lahtigui, D. Forster, C. Duchemin, N. Cramer, ACS Catal. 2022, 12, 6209–6215: “Enantioselective Access to 3-Azabicyclo[3.1.0]hexanes by CpxRhIII Catalyzed C–H Activation and Cp*IrIII Transfer Hydrogenation“

J. Klett, L. Wozniak, N. Cramer, Angew. Chem. Int. Ed. 2022, 61, e202202306: “1,3,2-Diazaphospholene-Catalyzed Reductive Cyclizations of Organohalides”
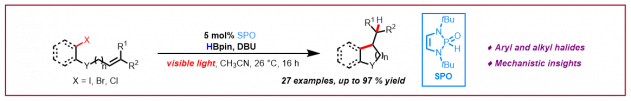
J.-F. Tan, C. T. Bormann, K. Severin, N. Cramer, Chem. Sci. 2022, 13, 3409-3415: “Chemo– and regio–divergent access to fluorinated 1–alkyl and 1–acyl triazenes from alkynyl triazenes”
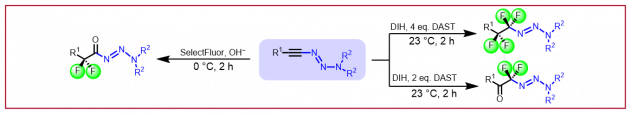
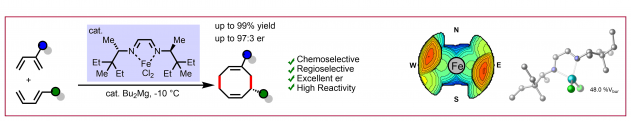
E. Braconi, N. Cramer, Angew. Chem. Int. Ed. 2022, 61, e202112148: “Crossed Regio- and Enantioselective Iron-Catalyzed [4+2]-Cycloadditions of Unactivated Dienes”

2021
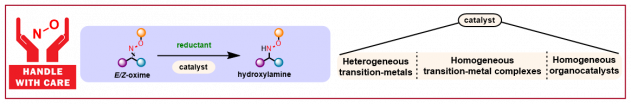
J. Mas-Rosello, N. Cramer, Chem. Eur. J. 2021, e202103683: “Catalytic reduction of oximes to hydroxylamines: current methods, challenges and opportunities”
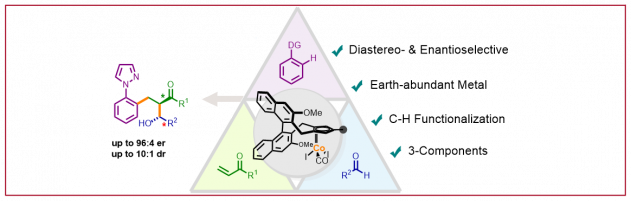
A. G. Herraiz, N. Cramer, ACS Catal. 2021, 11, 11938: “Cobalt(III)-Catalyzed Diastereo- and Enantioselective Three-Component C–H Functionalization”
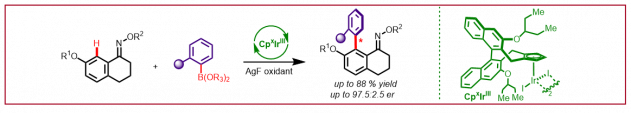
L. Wozniak, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 18532-18536: “Atropo-Enantioselective Oxidation-Enabled Iridium(III)-Catalyzed C–H Arylations with Aryl Boronic Esters”
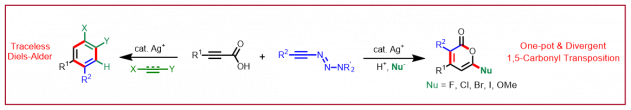
J.-F. Tan, C. T. Bormann, K. Severin, N. Cramer, Chem. Sci. 2021, 12, 9140-9145: “Alkynyl Triazenes Enable Divergent Syntheses of 2-Pyrones”
J. Mas-Rosello, C. J. Cope, E. Tan, B. Pinson, A. Robinson, T. Smejkal, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 15524–15532 : “Iridium-Catalyzed Acid-Assisted Hydrogenation of Oximes to Hydroxylamines”

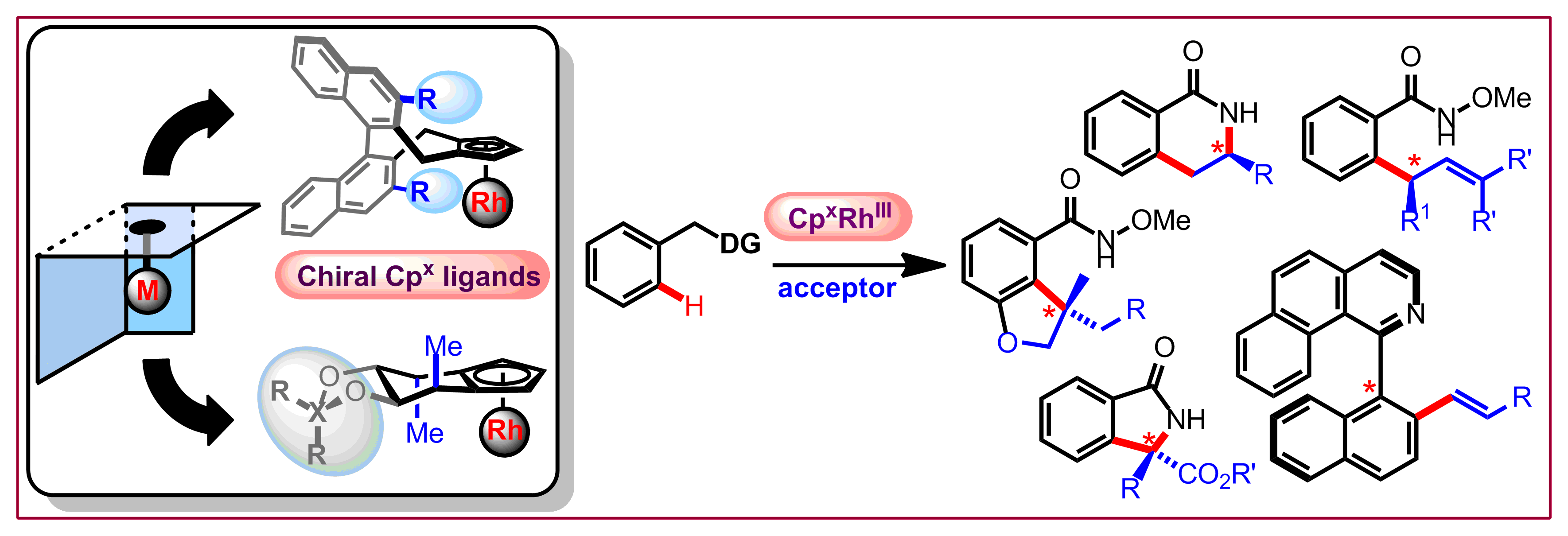
J. Mas-Roselló, A. G. Herraiz, B. Audic, A. Laverny, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 13198-13244: “Chiral cyclopentadienyl ligands: design, syntheses and applications in asymmetric catalysis”
K. Ozols, S. Onodera, L. Wozniak, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 655-659: “Cobalt(III)-Catalyzed Enantioselective Intermolecular Carboaminations via C-H Functionalizations”

2020
A. Laverny, N. Cramer, Organometallics 2020, 39, 4444-4456: “Accessing Monosubstituted Cyclopentadienyl Rhodium(I) andIridium(I) Complexes by a Simultaneous Nucleophilic Addition-Metalation Approach to Fulvenes”
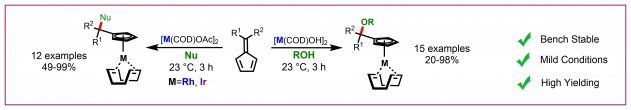
E. Braconi, A. C. Götzinger, N. Cramer, J. Am. Chem. Soc. 2020, 142, 19819-19824: “Enantioselective Iron-Catalyzed Cross-[4+4]-Cycloaddition of 1,3-Dienes Provides Chiral Cyclooctadienes”
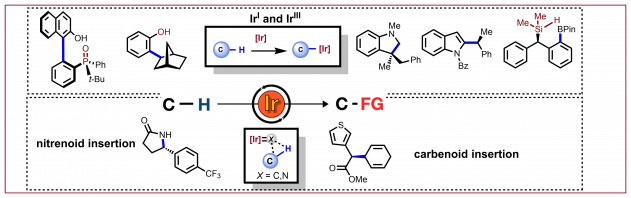
L. Wozniak, J.-F. Tan, Q.-H. Nguyen, A. Madron du Vigné, V. Smal, Y.-X. Cao, N. Cramer, Chem. Rev. 2020, 120, 10516−10543: “Catalytic Enantioselective Functionalizations of C−H Bonds by Chiral Iridium Complexes”
J. H. Reed, J. Klett, C. Steven, N. Cramer, Org. Chem. Front. 2020, 7, 3521–3529: “Stay positive: catalysis with 1,3,2-diazaphospholenes”
S.-G. Wang, N. Cramer, ACS Catal. 2020, 10, 8231-8236: “Asymmetric CpxRh(III)-Catalyzed Acrylic Acid C-H Functionalization with Allenes Provides Chiral γ-Lactones”
B. Audic, N. Cramer, Org. Lett. 2020, 22, 5030-5034: “Rhodium(III)-Catalyzed Cyclopropane C–H/C–C Activation Sequence Provides Diastereoselective Access to α-Alkoxylated γ-Lactams”
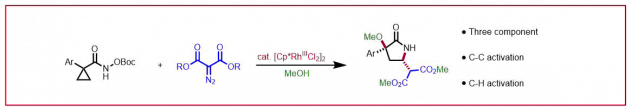
E. Braconi, N. Cramer, Angew. Chem. Int. Ed. 2020, 59, 16425-16429: “A Chiral Naphthyridine Diimine Ligand Platform Enables Nickel-Catalyzed Asymmetric Alkylidenecyclopropanations”

J. Mas-Rosello, T. Smejkal, N. Cramer, Science 2020, 368, 1098-1102: “Iridium-catalyzed acid-assisted asymmetric hydrogenation of oximes to hydroxylamines”
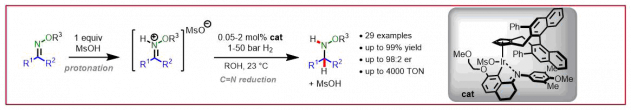
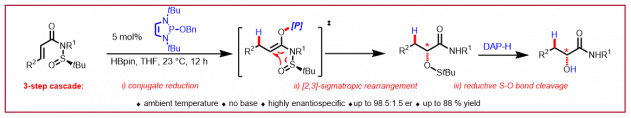
J. H. Reed, N. Cramer, ChemCatChem 2020, 12, 4262-4266: “1,3,2‐Diazaphospholenes Catalyze the Conjugate Reduction of Substituted Acrylic Acids”
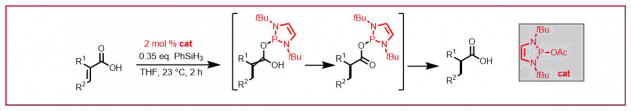
C. Duchemin, N. Cramer, Angew. Chem. Int. Ed. 2020, 59, 14129-14133: “Enantioselective CpxRhIII–Catalyzed Carboaminations of Acrylates”
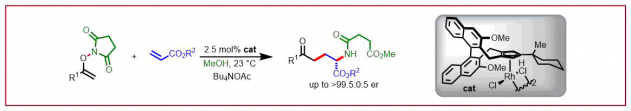
J. Diesel, N. Cramer, Chimia 2020, 74, 278-284: “Modular Chiral N-Heterocyclic Carbene Ligands for the Nickel-Catalyzed Enantioselective C–H Functionalization of Heterocycles”
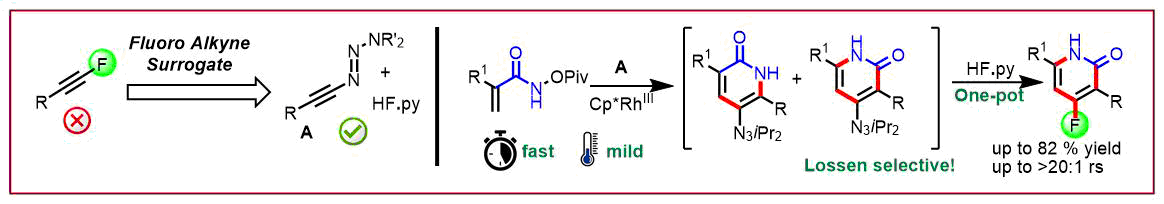
J.-F. Tan, C. T. Bormann, K. Severin, N. Cramer, ACS Catalysis 2020, 10, 3790-3796: “Alkynyl Triazenes as Fluoroalkyne Surrogates: Regioselective Access to 4-Fluoro-2-pyridones by a Rh(III)-Catalyzed C-H Activation-Lossen Rearrangement-Wallach Reaction”
Q.-H. Nguyen, S.-M. Guo, T. Royal, O. Baudoin, N. Cramer, J. Am. Chem. Soc. 2020, 142, 2161-2167: “Intermolecular Palladium(0)-Catalyzed Atropo-enantioselective C–H Arylation of Heteroarenes”

2019
S. H. Park, S.-G. Wang, N. Cramer, ACS Catal. 2019, 9, 10226-10231: “Enantioselective Ruthenium(II)-Catalyzed Access to Benzonorcaradienes by Coupling of Oxabenzonorbornadienes and Alkynes”

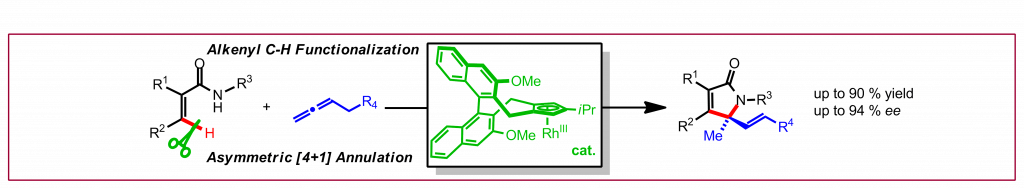
S.-G. Wang, Y. Liu, N. Cramer, Angew. Chem. Int. Ed. 2019, 58, 18136-18140: “Asymmetric Alkenyl C-H Functionalization by CpxRhIII forms 2H-Pyrrol-2-ones by [4+1]-Annulation of Acryl Amides and Allenes”
J. Diesel, N. Cramer, ACS Catal. 2019, 9, 9164−9177: “Generation of Heteroatom Stereocenters by Enantioselective C–H Functionalization”
C. Duchemin, G. Smits, N. Cramer, Organometallics 2019, 38, 3939-3947: “RhI, IrIII and CoIII Complexes with Atropchiral Biaryl Cyclopentadienyl Ligands: Syntheses, Structures and Catalytic Activities”
J.-F. Tan, C. T. Bormann, F. G. Perrin, F. M. Chadwick, K. Severin, N. Cramer, J. Am. Chem. Soc. 2019, 141, 10372: “Divergent Synthesis of Densely Substituted Arenes and Pyridines via Cyclotrimerization Reactions of Alkynyl Triazenes”
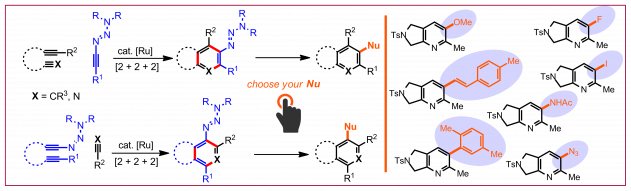
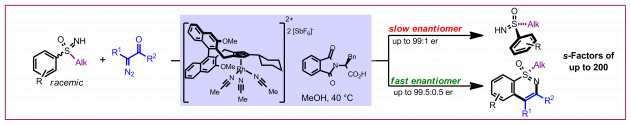
M. Brauns, N. Cramer, Angew. Chem. Int. Ed. 2019, 58, 8902-8906: “Efficient Kinetic Resolution of Sulfur-Stereogenic Sulfoximines Exploiting CpxRhIII-Catalyzed C-H Functionalization”
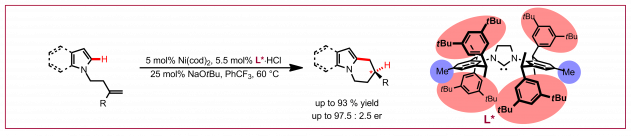
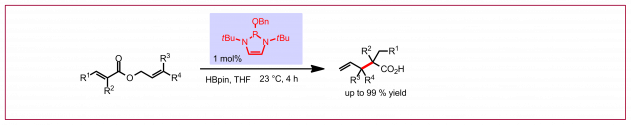
Ł. Woźniak, N. Cramer, Trends Chem. 2019, 1, 471-484: “Enantioselective C–H Bond Functionalizations by 3d Transition-Metal Catalysts“

D. Grosheva, N. Cramer, Chimia 2019, 73, 262-267: “Exploitation of Unconventional Electrophiles in Enantioselective Pd(0)-Catalyzed C-H Functionalizations“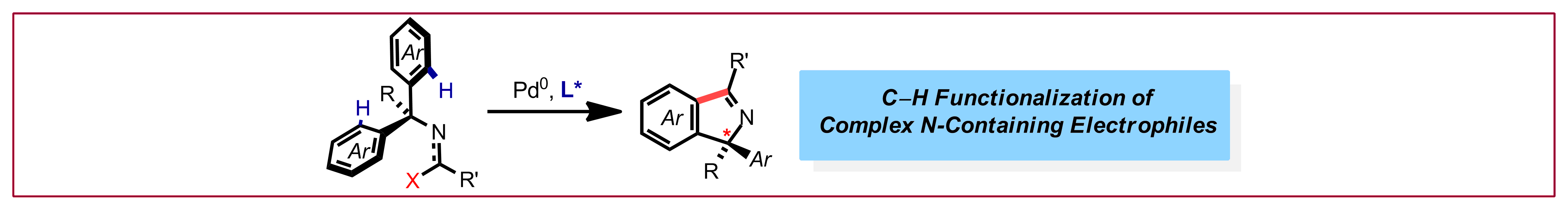
K. Ozols, Y.-S. Jang, N. Cramer, J. Am. Chem. Soc. 2019, DOI:10.1021/jacs.9b02569: “Chiral Cyclopentadienyl Cobalt(III) Complexes Enable HighlyEnantioselective 3d-Metal-Catalyzed C-H Functionalizations”
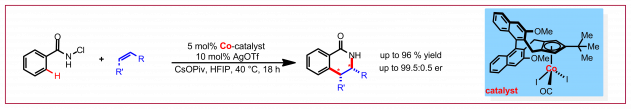
C. Duchemin, N. Cramer, Chem. Sci. 2019, 10, 2773–2777: “Chiral Cyclopentadienyl RhIII-Catalyzed Enantioselective Cyclopropanation of Electron-Deficient Olefins Enable Rapid Access to UPF-648 and Oxypilin Natural Products“

S.-G. Wang, N. Cramer, Angew. Chem. Int. Ed. 2019, 58, 2514–2518: “An Enantioselective CpxRh(III)–Catalyzed C–H Functionalization / Ring–Opening Route to Chiral Cyclopentenylamines”
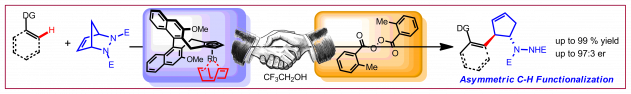
C. Duchemin, N. Cramer, Org. Chem. Front. 2019, 6, 209-212: “One-step access to N-enoxyimides by gold-catalysed addition of N-hydroxyimides to terminal alkynes”

2018
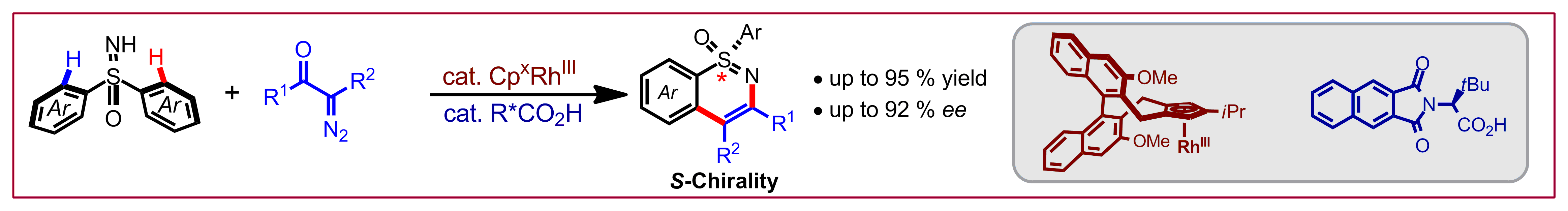
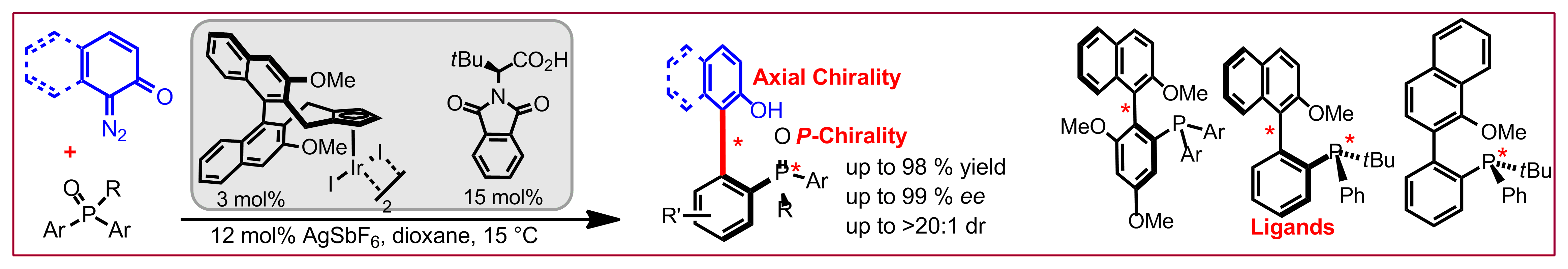
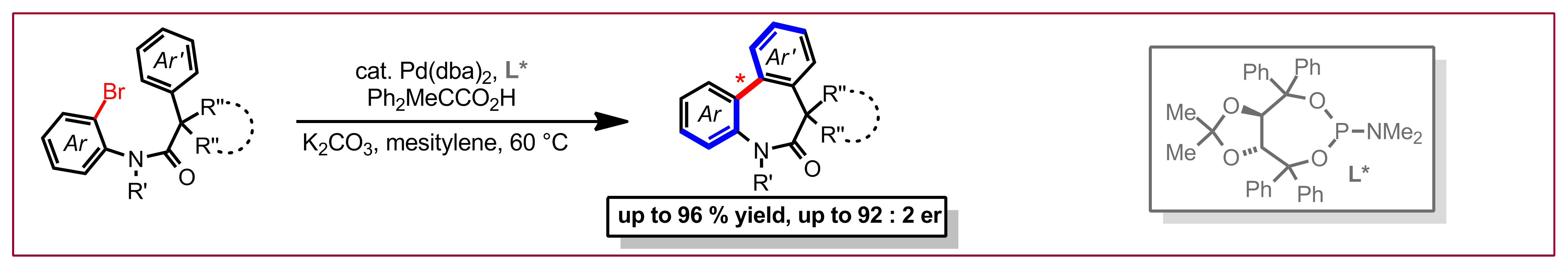
J. Diesel, A. Finogenova, N. Cramer, J. Am. Chem. Soc. 2018, 140, 4489-4493: “Nickel-Catalyzed Enantioselective Pyridone C-H Functionalizations Enabled by a Bulky N-Heterocyclic Carbene Ligand”
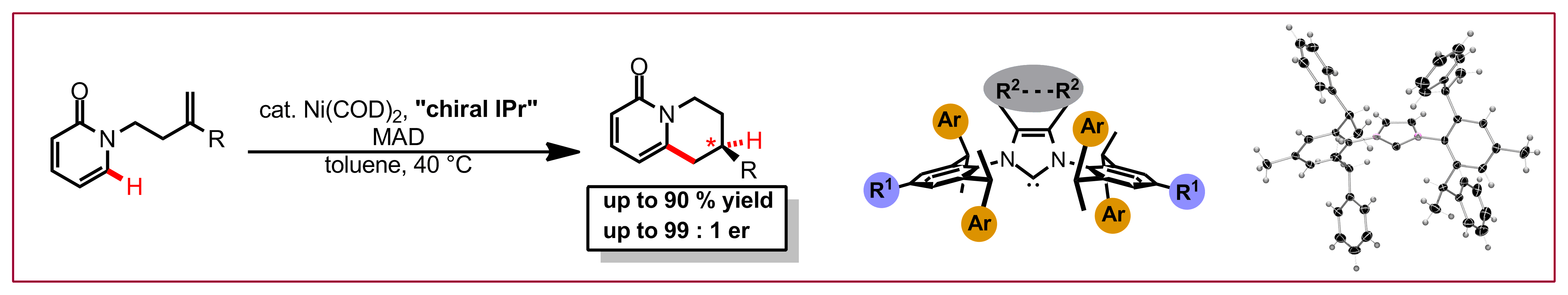
S.-G. Wang, S. H. Park, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 5459-5462: “A Readily Accessible Class of Chiral Cp Ligands and their Application in Ru(II)-Catalyzed Enantioselective Syntheses of Dihydrobenzoindoles”
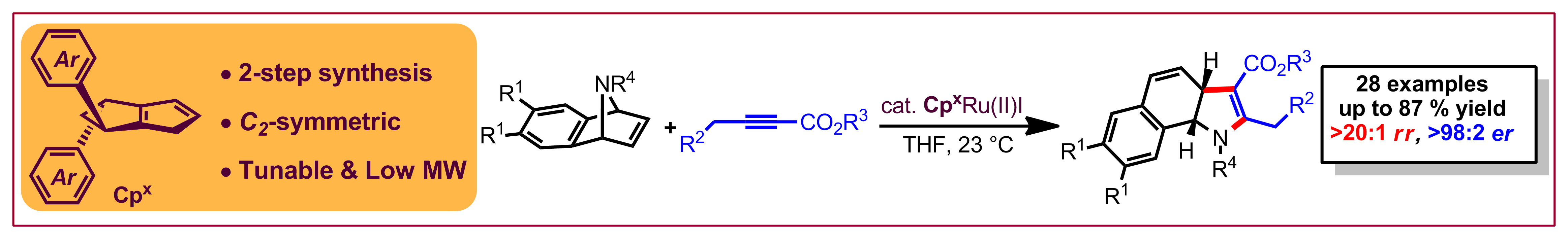
S. Miaskiewicz, J. H. Reed, P. A. Donets, C. C. Oliveira, N. Cramer, Angew. Chem. Int. Ed. 2018,
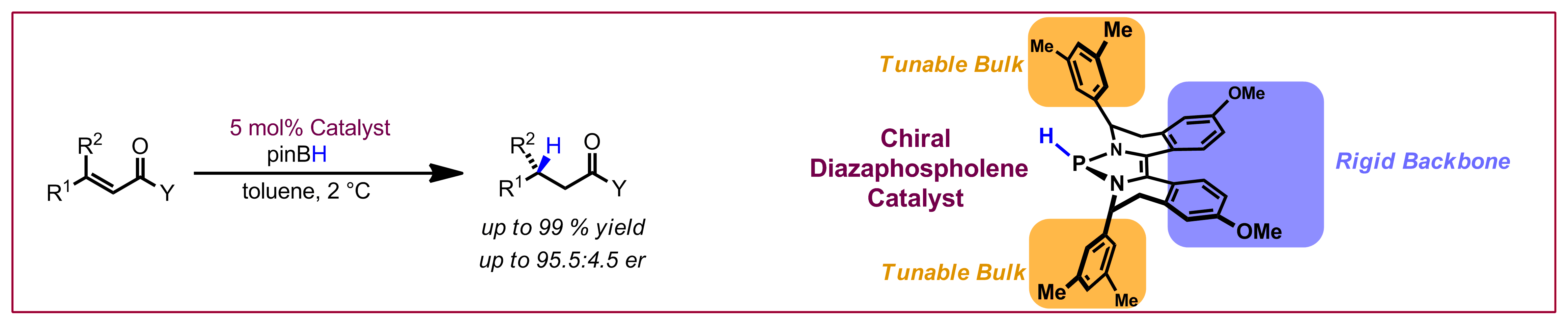
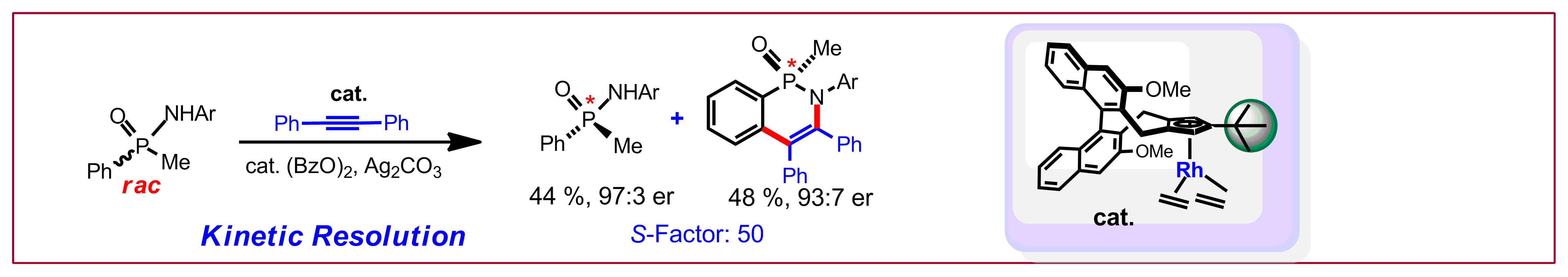
Y. Sun, N. Cramer, Chem. Sci. 2018, 9, 2981-2985: “Tailored trisubstituted chiral CpxRhIII catalysts for kinetic resolutions of phosphinic amides”
2017
D. Grosheva, N. Cramer, ACS Catalysis 2017, 7, 7417-7420: “Ketene Aminal Phosphates: Competent Substrates for Enantioselective Pd(0)-Catalyzed C-H Functionalizations”
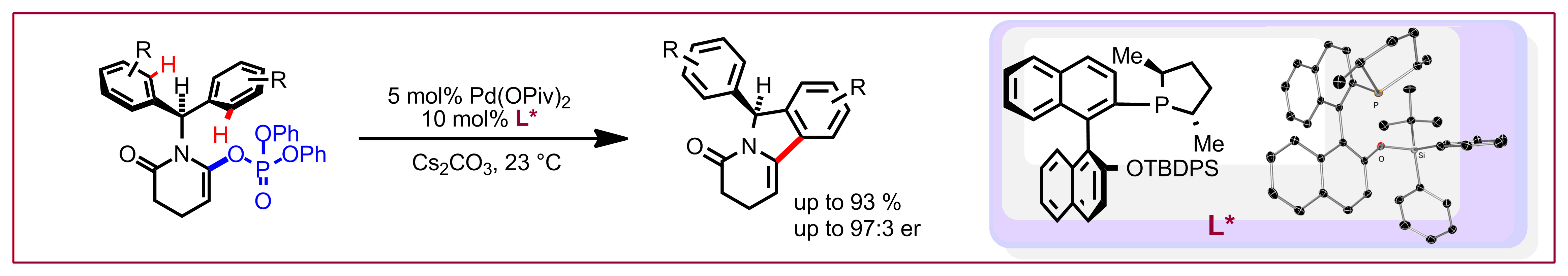
Y.-S. Jang, M. Dieckmann, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 15088-15092: “Cooperative Effects between Chiral CpxIrIII Catalysts and Chiral Carboxylic Acids in Enantioselective C-H Amidations of Phosphine Oxides”
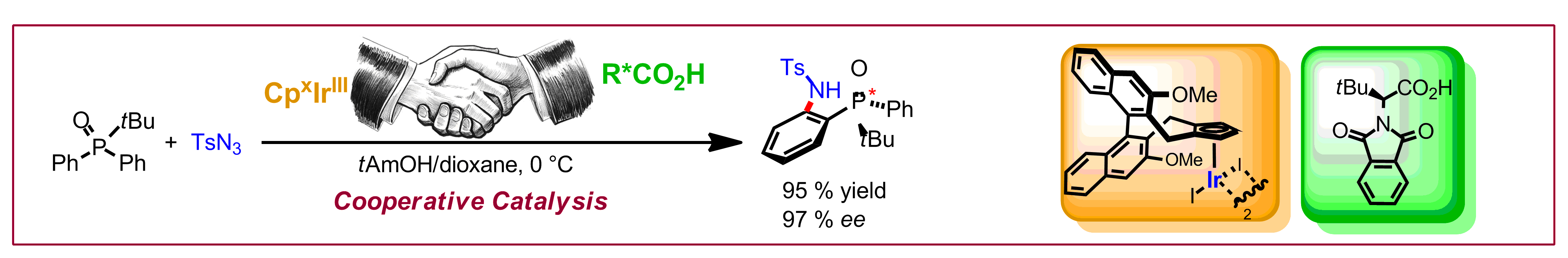
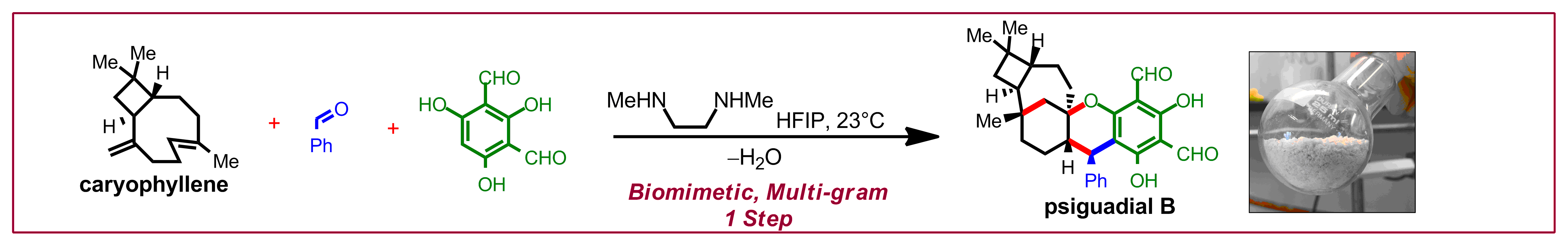
C. G. Newton, D. N. Tran, M. D. Wodrich, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 13776-13780: “One-step Multigram-scale Biomimetic Synthesis of Psiguadial B”
J. Pedroni, N. Cramer, J. Am. Chem. Soc. 2017, 139, 12398-12401: “Enantioselective C–H Functionalization–Addition Sequence Delivers Densely Substituted 3-Azabicyclo[3.1.0]hexanes”
G. Smits, B. Audic, M. D. Wodrich, C. Corminboeuf, N. Cramer, Chem. Sci. 2017, 8, 7174-7179: “A β-Carbon elimination strategy for convenient in situ access to cyclopentadienyl metal complexes”
D. Kossler, F. G. Perrin, A. A. Suleymanov, G. Kiefer, R. Scopelliti, K. Severin, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 11490-11493: “Divergent Asymmetric Synthesis of Polycyclic Compounds via Vinyl Triazenes”
D. Kossler, N. Cramer, Chimia 2017, 71, 186-189: “Chiral Cyclopentadienyl Ruthenium Complexes as Versatile Catalysts for Enantioselective Transformations”
C. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908-8976: “Catalytic Enantioselective Transformations Involving C-H Bond Cleavage by Transition-Metal Complexes”
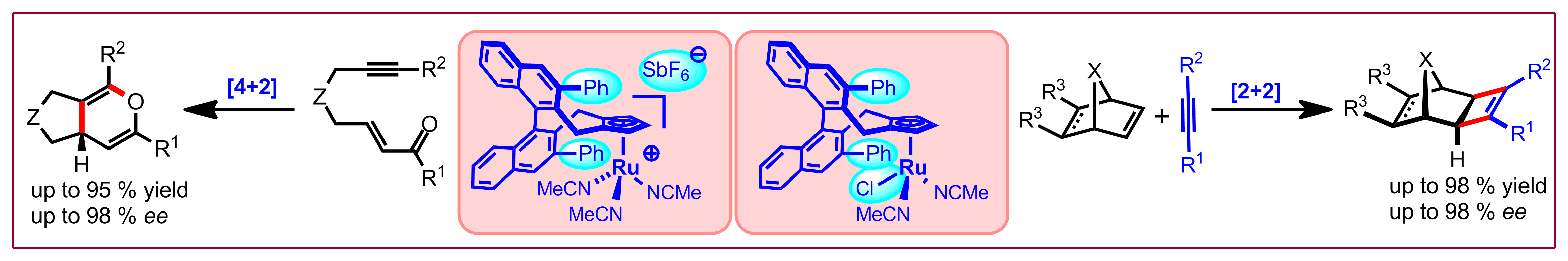
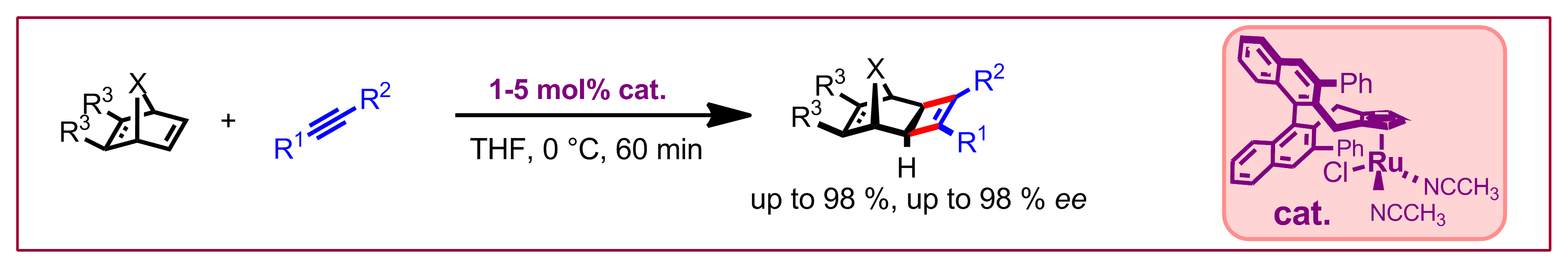
D. Kossler, N. Cramer, Chem. Sci. 2017, 8, 1862-1866: “Neutral Chiral Cyclopentadienyl Ru(II)Cl Catalysts Enable Enantioselective [2+2]-Cycloadditions”
Y. Sun, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 364-367: “Rhodium(III)-Catalyzed Enantiotopic C-H Activation Enables Access to P-Chiral Cyclic Phosphinamides”
2016
N. Cramer, Chem 2016, 1, 522-523: “Designer Anhydride Clears the Path for Alkoxycarbonylations in Catellani Reactions”

C. M. B. K. Kourra, N. Cramer, Chem. Science, 2016, 7, 7007-7012: “Converting Disulfide Bridges in Native Peptides to Stable Methylene Thioacetals”
J. S. E. Ahlin, N. Cramer, Org. Lett. 2016, 18, 3242-3245: “Chiral N‑Heterocyclic Carbene Ligand Enabled Nickel(0)-Catalyzed Enantioselective Three-Component Couplings as Direct Access to Silylated Indanols”
C. Heinz, N. Cramer, Chimia 2016, 70, 258: “Total Synthesis of Fijiolide A”

J. Pedroni, N. Cramer, Org. Lett. 2016, 18, 1932: “2-(Trifluoromethyl)indoles via Pd(0)-Catalyzed C(sp3)H Functionalization of Trifluoroacetimidoyl Chlorides”
C. G. Newton, D. Kossler, N. Cramer, J. Am. Chem. Soc. 2016, 138, 3935-3941: “Asymmetric Catalysis Powered by Chiral Cyclopentadienyl Ligands”
M. V. Pham, N. Cramer, Chem. Eur. J. 2016, 22, 2270-2273: “Enantioselective Access to Spirocyclic Sultams by Chiral Cpx-Rhodium(III)-Catalyzed Annulations”
2015
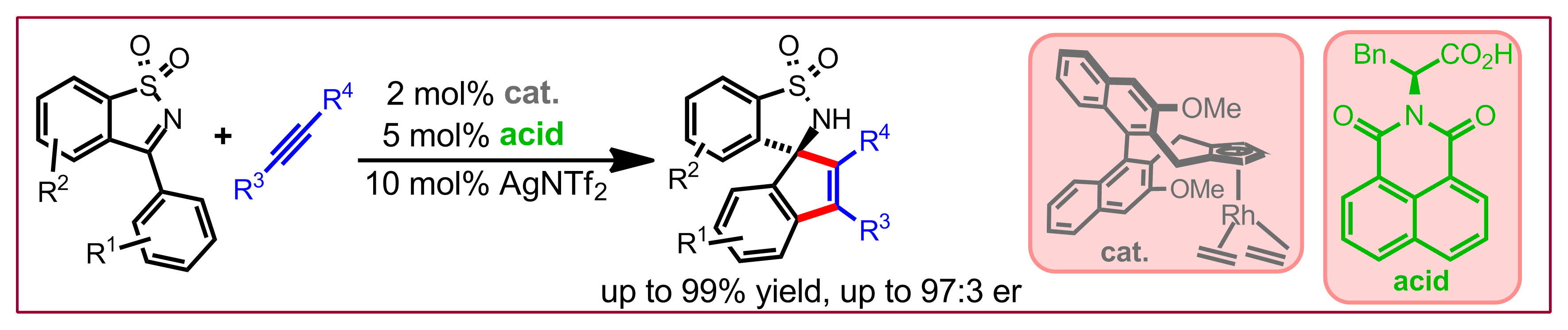
J. Pedroni, N. Cramer, Chem. Commun. 2015, 51, 17647-17657: “TADDOL-based Phosphorus(III)-Ligands in Enantioselective Pd(0)-Catalysed C-H Functionalisations”
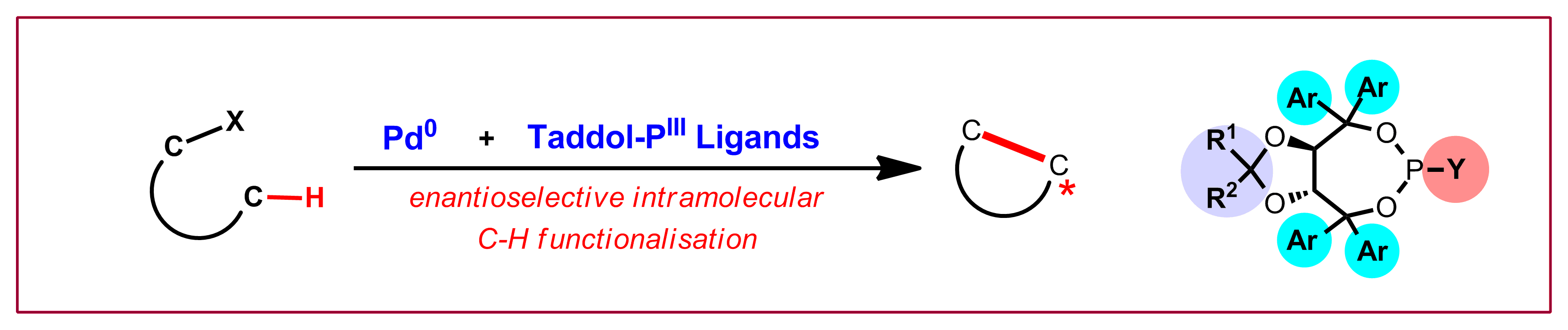
D. Kossler, N. Cramer, J. Am. Chem. Soc. 2015, 137, 12478: “Chiral Cationic CpxRu(II) Complexes for Enantioselective Yne-Enone Cyclizations”
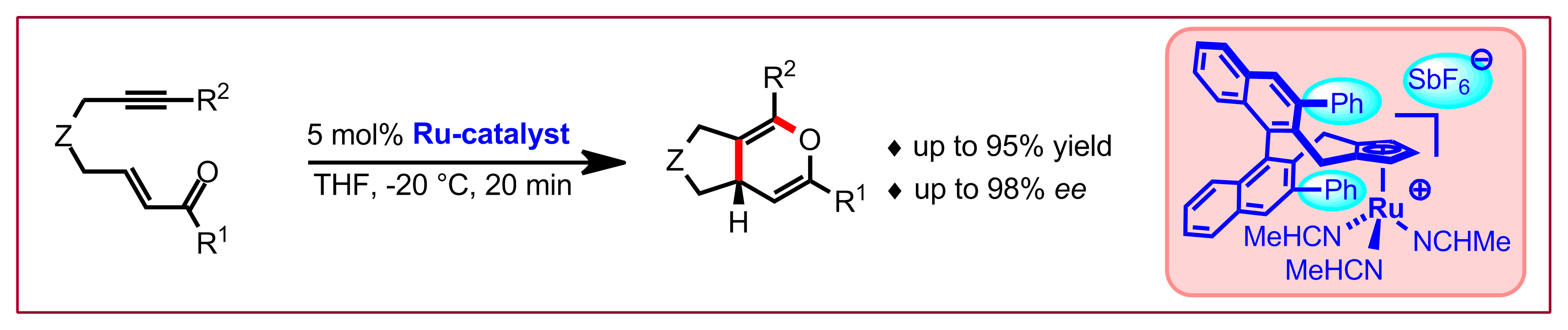
C. Heinz, N. Cramer, J. Am. Chem. Soc. 2015, 137, 11278-11281: “Synthesis of Fijiolide A via an Atropselective Paracyclophane Formation”
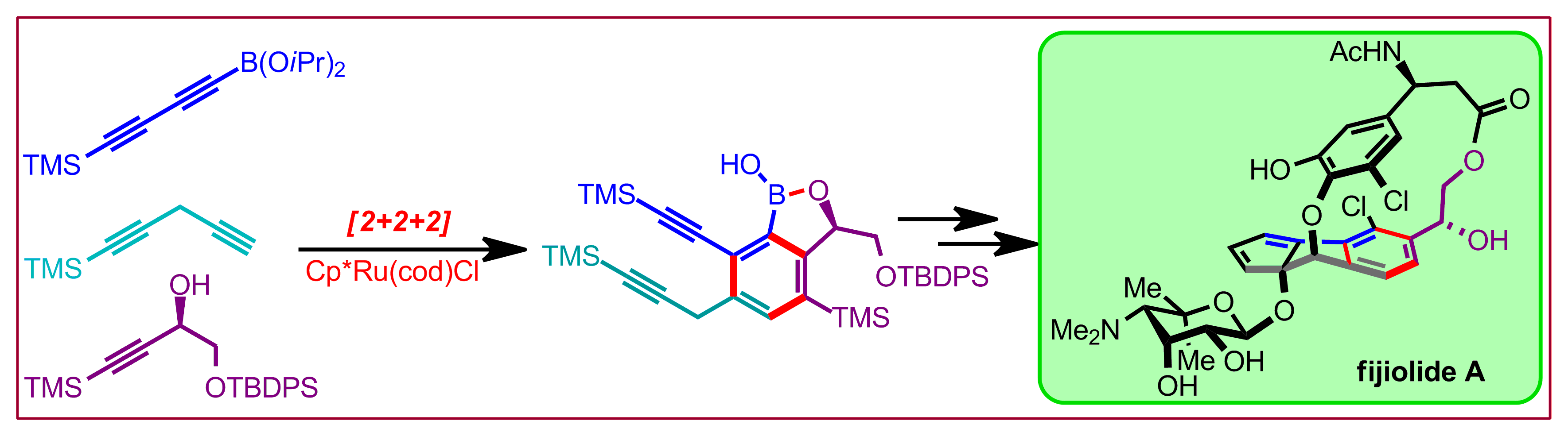
M. Dieckmann, Y.-S. Jang, N. Cramer, Angew. Chem. Int. Ed. 2015, 54, 12149-12152: “Chiral Cyclopentadienyl Iridium(III) Complexes Promote Enantioselective Cycloisomerizations Giving Fused Cyclopropanes”
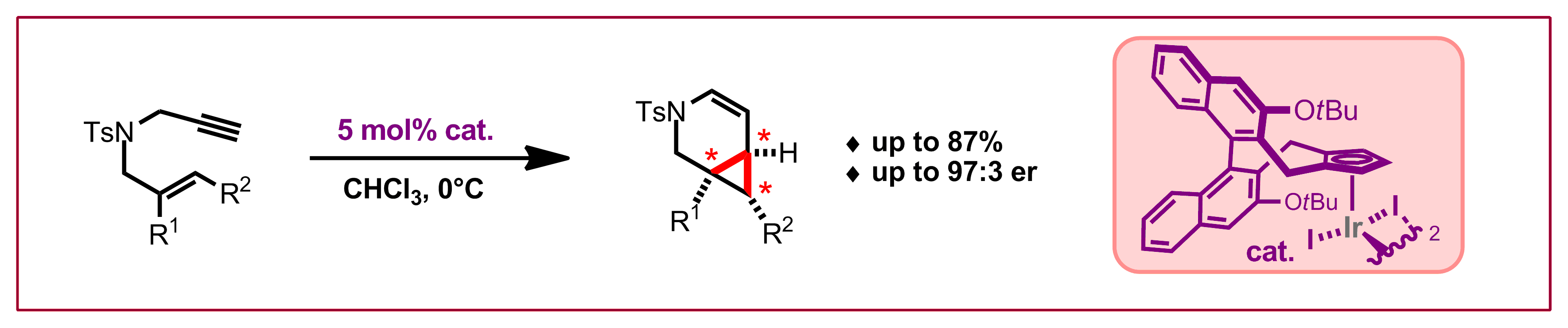
J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2015, 54, 11826-11829: “Chiral Gamma-Lactams by Enantioselective Palladium(0)-Catalyzed Cyclopropane Functionalizations”

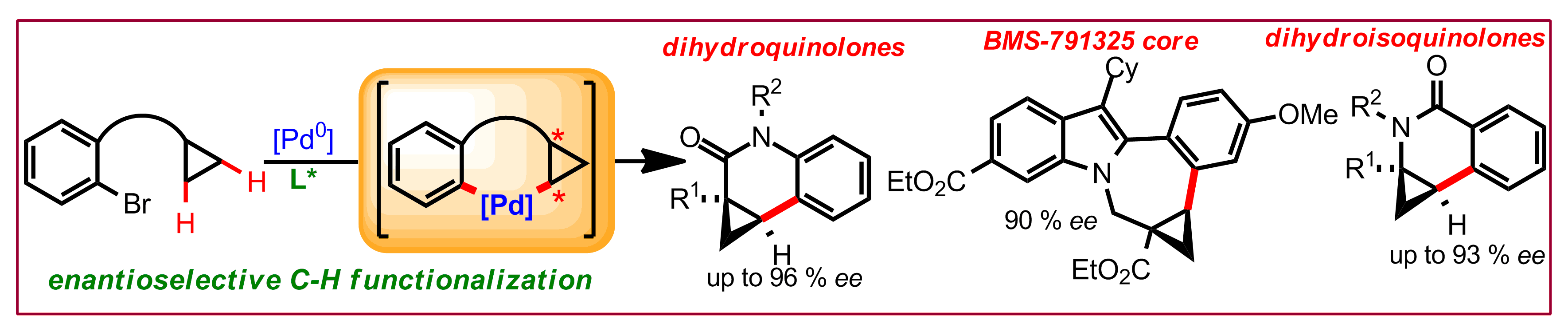
J. Pedroni, T. Saget, P. A. Donets, N. Cramer, Chem. Sci. 2015, 6, 5164-5171: “Enantioselective Palladium(0)‐Catalyzed Intramolecular Cyclopropane Functionalizations: Access to Dihydroquinolones, Dihydroisoquinolones and the BMS‐791325 Ring System”
L. Souillart, N. Cramer, Chem. Rev. 2015, 115, 9410-9464: “Catalytic C-C Bond Activations via Oxidative Addition to Transition Metals”
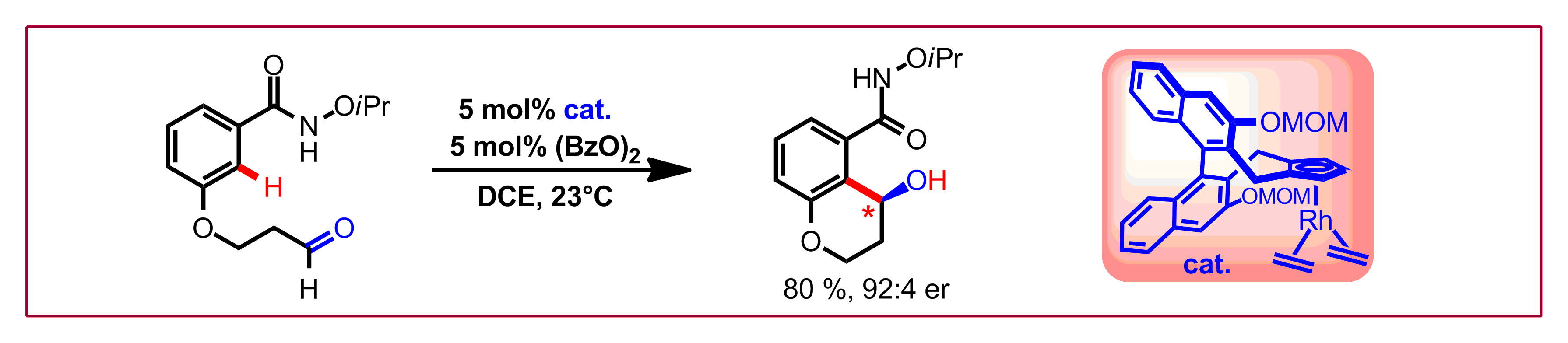
B. Ye, N. Cramer, Synlett 2015, 26, 1490-1495: “Chiral Cyclopentadienyl Ligands Enable a Rhodium(III)-Catalyzed Enantioselective Access to Hydroxychromames and Phthalides”
L. Souillart, N. Cramer, Chimia 2015, 69, 187-190: “Enantioselective Rhodium-catalyzed C-C Bond Activation of Cyclobutanones”

B. Ye, N. Cramer, Acc. Chem. Res. 2015, 48, 1308-1318: “Chiral Cyclopentadienyls: Enabling Ligands for Asymmetric Rh(III)-Catalyzed C-H Functionalizations”
C. M. B. K. Kourra, N. Cramer, Nature 2015, 517, 440-441: “Gold Unleashes the Power of Three, News & Views”

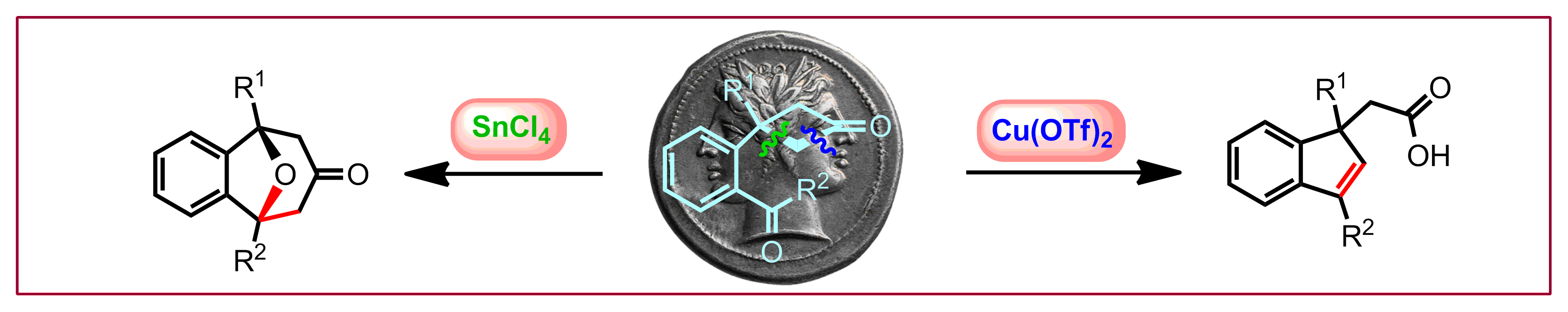
L. Souillart, N. Cramer, Chem. Eur. J. 2015, 21, 1863-1867: “Regiodivergent Cyclobutanone Cleavage: Switching Selectivity with Different Lewis Acids”
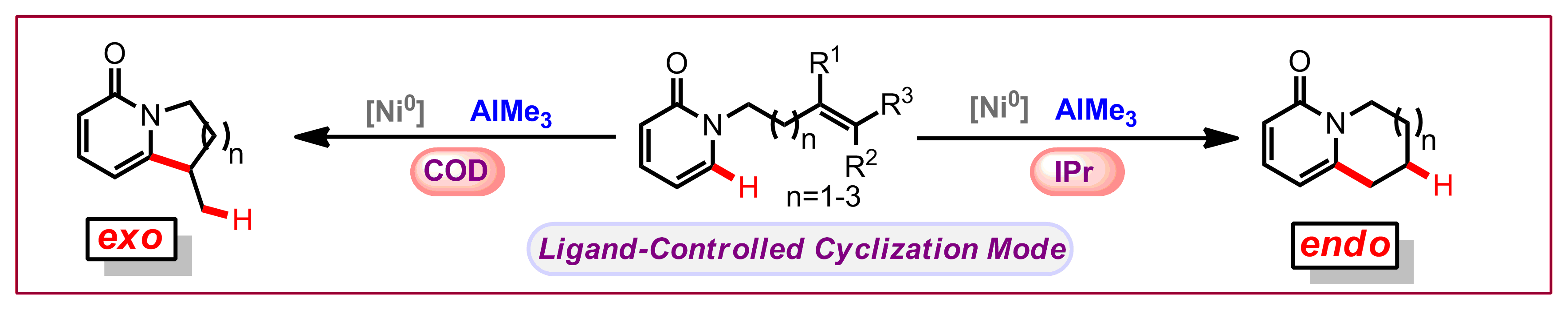
P. A. Donets, N. Cramer, Angew. Chem. Int. Ed. 2015, 54, 633-637: “Ligand-Controlled Regiodivergent Nickel-Catalyzed Annulation of Pyridones”
2014
M. V. Pham, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 14575-14579: “Rhodium(III)/Copper(II)-Promoted trans-Selective Heteroaryl Acyloxylation of Alkynes: Stereodefined Access to trans-Enol Esters” 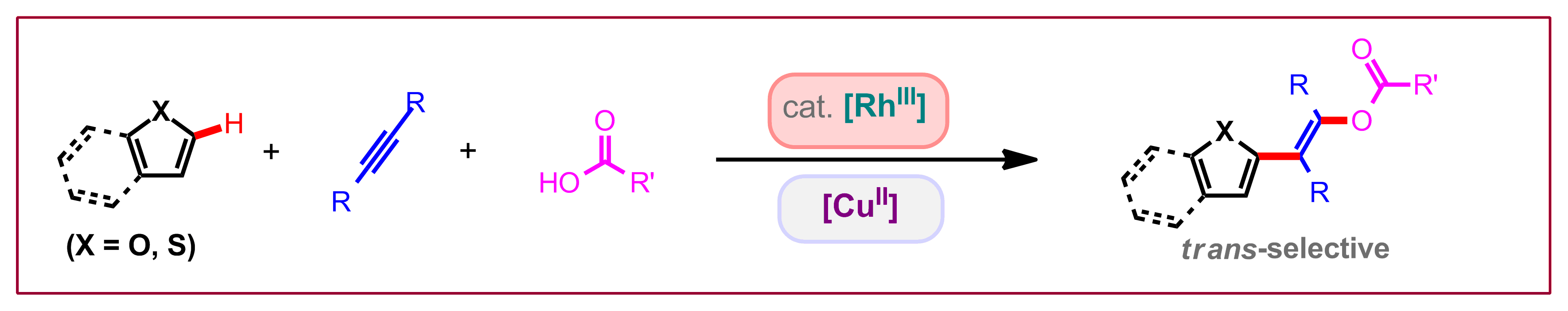
T. Saget, N. Cramer, e-EROS Encyclopedia of Reagents for Organic Synthesis 2014, DOI: 10.1002/047084289X.rn01777: “(1R,7R)-4-Dimethylamino-9,9-dimethyl-2,2,6,6-tetrakis(3,5-dimethylphenyl)-3,5,8,10-tetraoxa-4-phosphabicyclo[5.3.0]decane”

M. D. Wodrich, B. Ye, J. F. Gonthier, C. Corminboeuf, N. Cramer, Chem. Eur. J. 2014, 20, 15409-15418: “Ligand-Controlled Regiodivergent Pathways of Rhodium(III)-Catalyzed Dihydroisoquinolone Synthesis: Experimental and Computational Studies of Different Cyclopentadienyl Ligands”
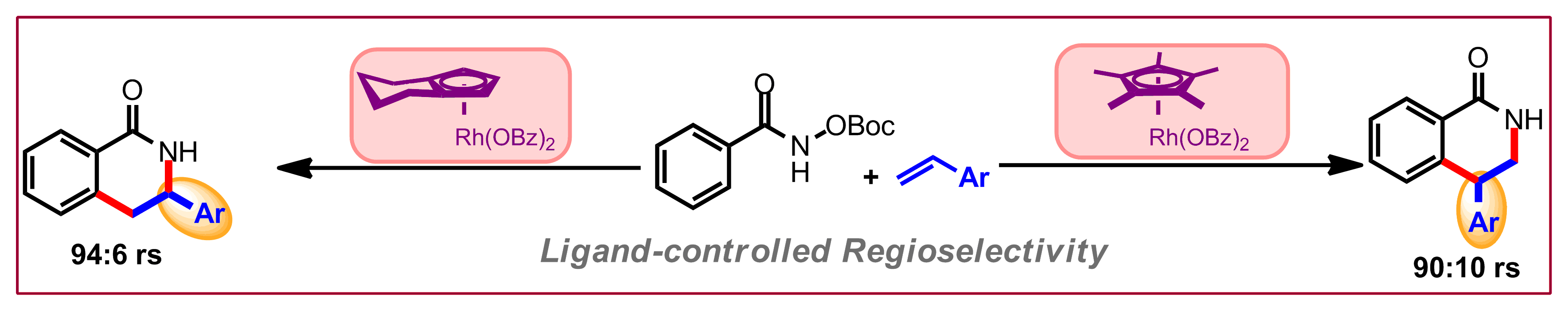
J. S. E. Ahlin, P. A. Donets, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 13229-13233: “Nickel(0)-Catalyzed Enantioselective Annulations of Alkynes and Arylenoates Enabled by a Chiral NHC Ligand: Efficient Access to Cyclopentenones”
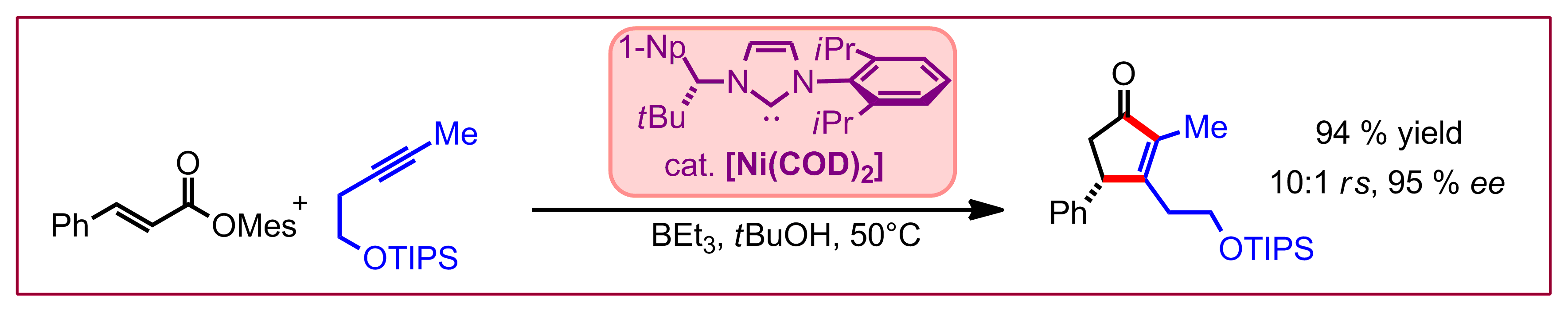
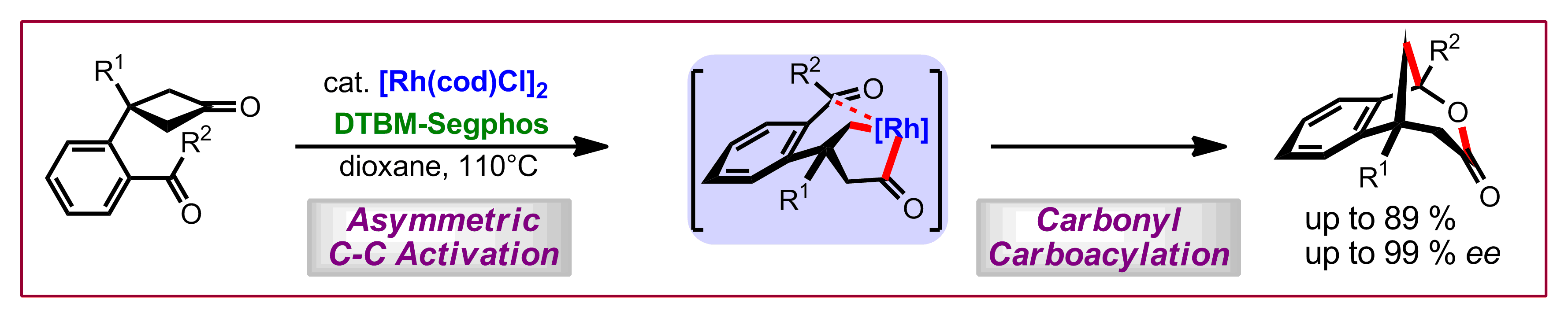
L. Souillart, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 9640-9644: “Highly Enantioselective Rhodium(I)-Catalyzed Carbonyl Carboacylations Initiated by C-C Bond Activation”
J. Pedroni, M. Boghi, T. Saget, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 9064-9067: “Access to β-Lactams by Enantioselective Palladium(0)-Catalyzed C(sp3)-H Alkylation”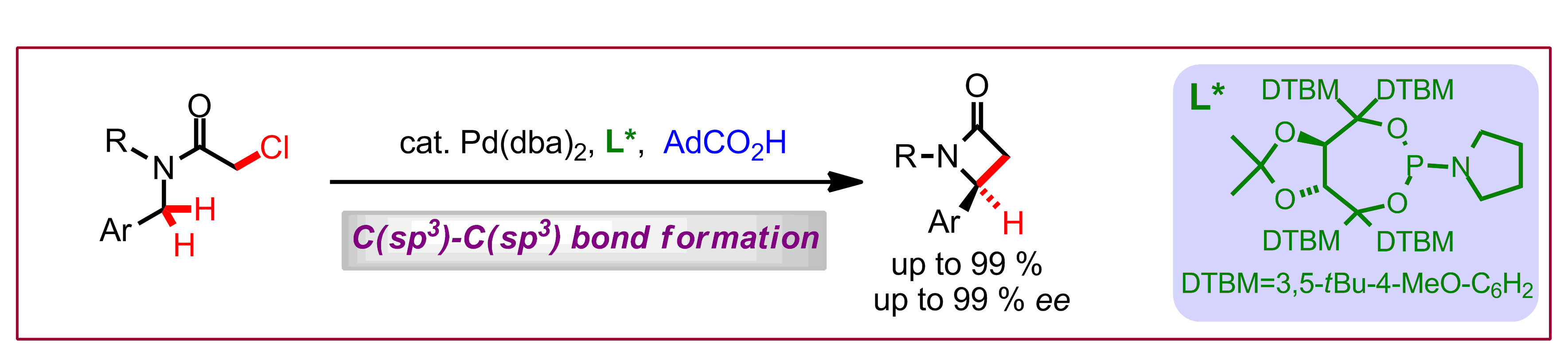
B. Ye, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 7896-7899: “Asymmetric Synthesis of Isoindolones by Chiral Cyclopentadienyl-Rhodium(III)-Catalyzed C-H Functionalizations”
D. N. Tran, N. Cramer, Chem. Eur. J. 2014, 20, 10654-10660: “Biomimetic Synthesis of (+)-Ledene, (+)-Viridiflorol, (-)-Palustrol, (+)-Spathulenol, Psiguadial A, C and D via the Platform Terpene (+)-Bicyclogermacrene”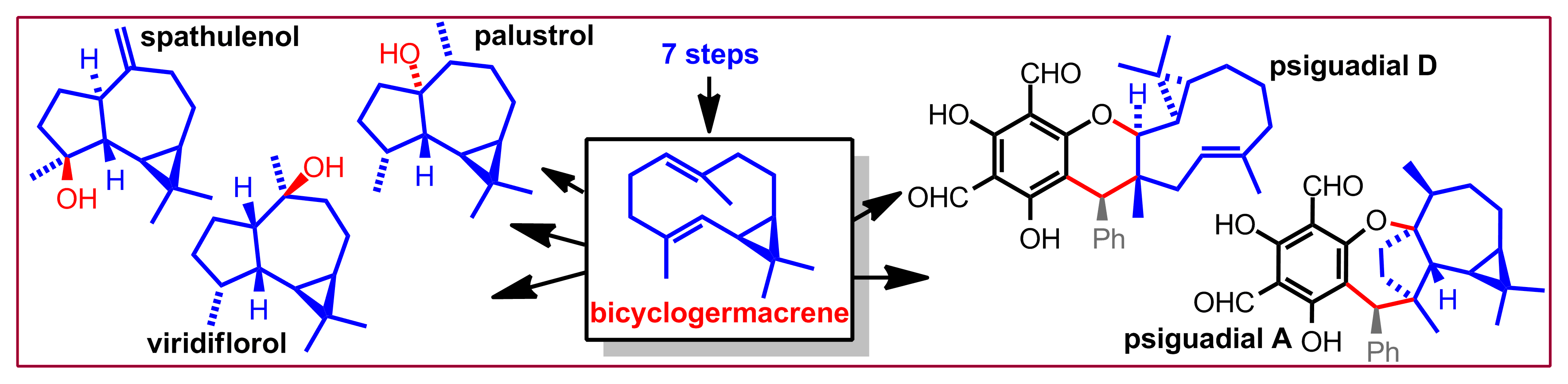
T. Saget, N. Cramer, Pure Appl. Chem. 2014, 86, 265-272: “Enantioselective Palladium(0)-Catalyzed C-H Arylation Strategy for Chiral Heterocycles”

L. Souillart, E. Parker, N. Cramer Top. Curr. Chem. 2014, DOI: 10.1007/128-2013_505: “Asymmetric Transformations via C-C Bond Cleavage”

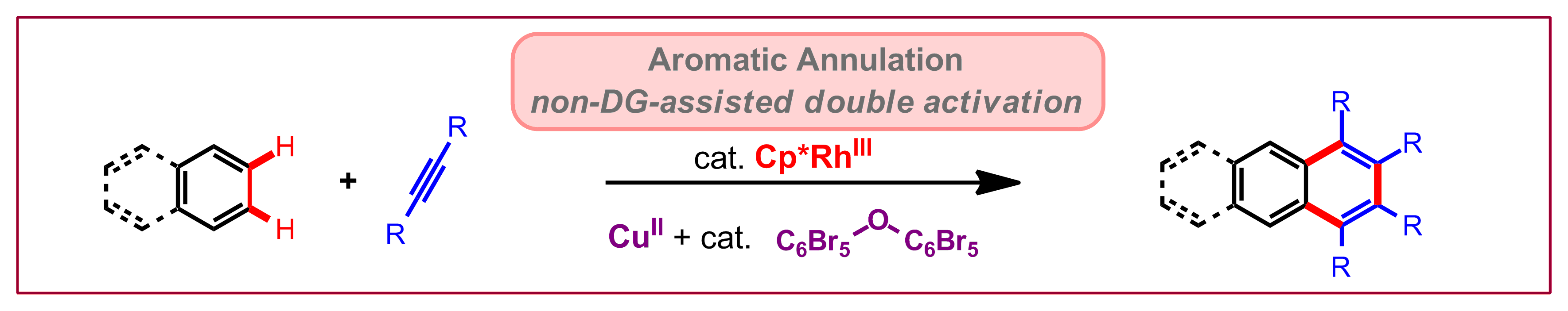
M. V. Pham, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 3484-3487: “Aromatic Homologation by Non-Chelate-Assisted RhIII-Catalyzed C-H Functionalization of Arenes with Alkynes”
L. Souillart, E. Parker, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 3001-3005: “Highly Enantioselective Rhodium(I)-Catalyzed Activation of Enantiotopic Cyclobutanone C-
2013
B. M. Trost, D. A. Bringley, T. Zhang, N. Cramer, J. Am. Chem. Soc. 2013, 135, 16720-16735: “Rapid Access to Spirocyclic Oxindole Alkaloids: Application of the Asymmetric Palladium-Catalyzed [3 + 2] Trimethylenemethane Cycloaddition“
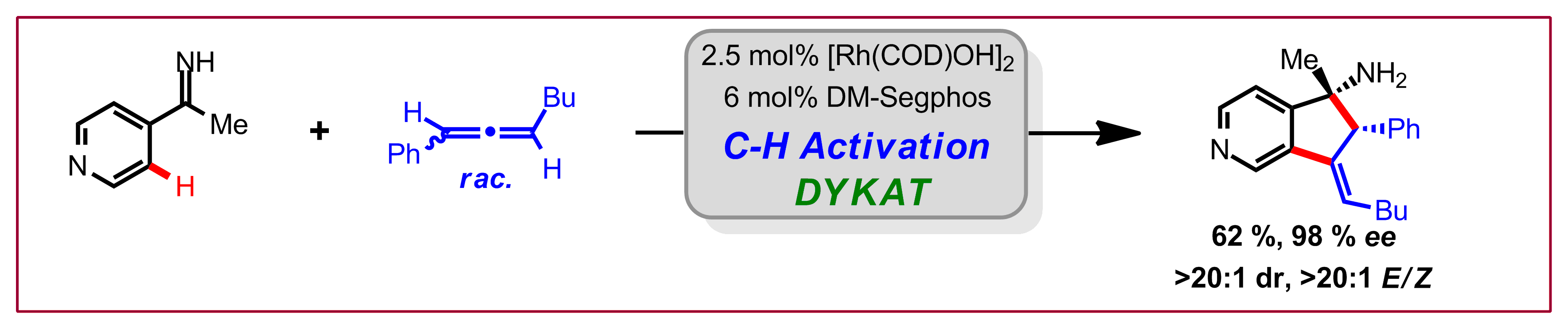
D. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2013, 52, 10630-10634: “Rhodium-Catalyzed Dynamic Kinetic Asymmetric Transformations of Racemic Allenes by the [3+2] Annulationof Aryl Ketimines”
P. A. Donets, N. Cramer, J. Am. Chem. Soc. 2013, 135, 11772-11775: “Diaminophosphine Oxide Ligand Enabled Asymmetric Nickel-Catalyzed Hydrocarbamoylations of Alkenes”
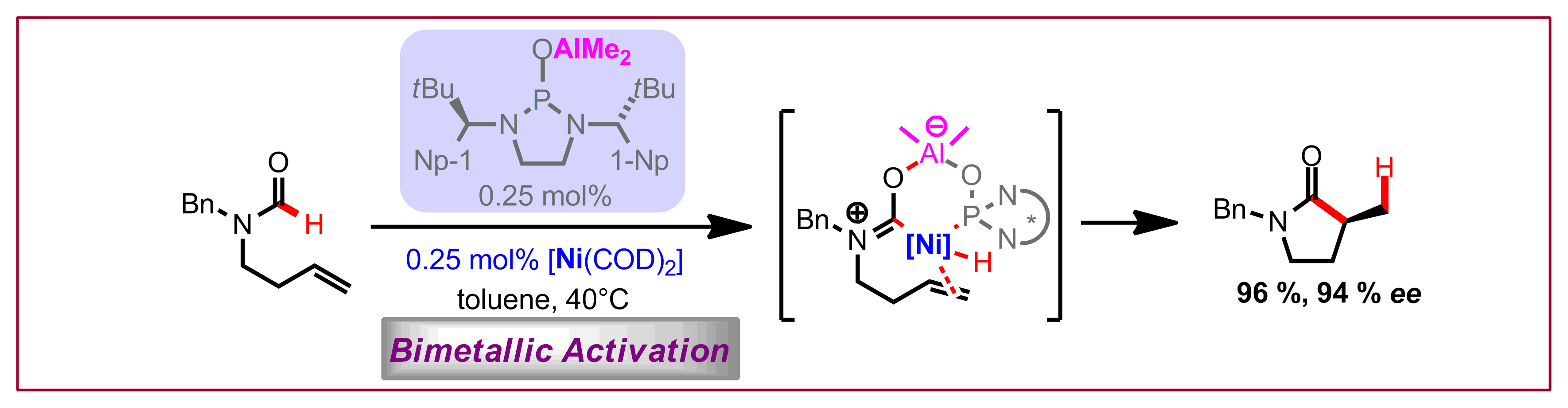
T. Saget, N. Cramer, Angew. Chem. Int. Ed. 2013, 52, 7865-7868: “Enantioselective C-H Arylation Strategy for Functionalized Dibenzazepinones with Quaternary Stereocenters”
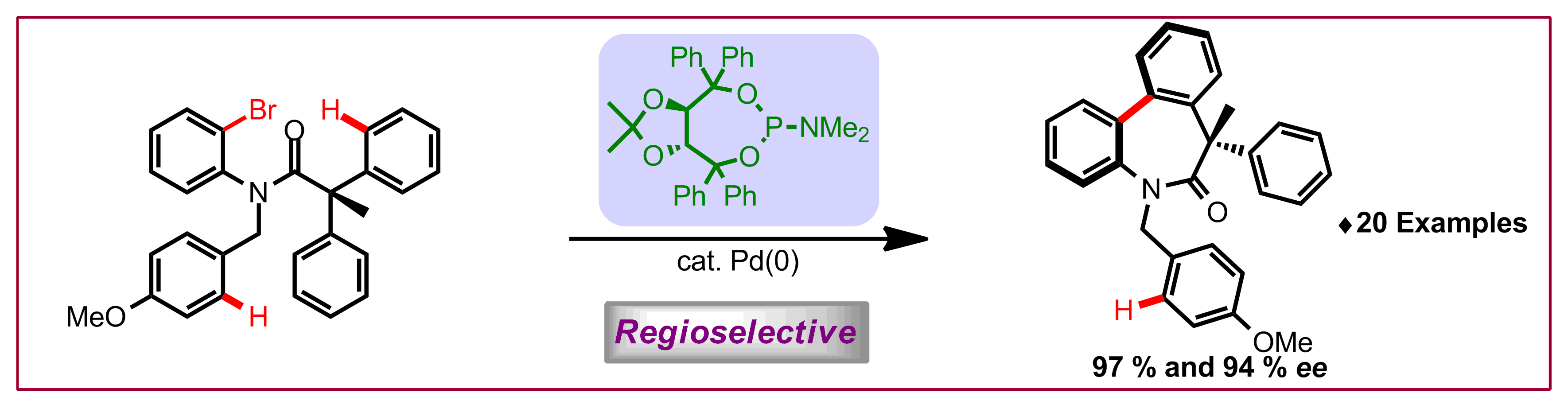
T. Saget, D. Perez, N. Cramer, Org. Lett. 2013, 15, 1354-1357: “Synthesis of Functionalized Spiroindolines via Palladium-Catalyzed Methine C–H Arylation”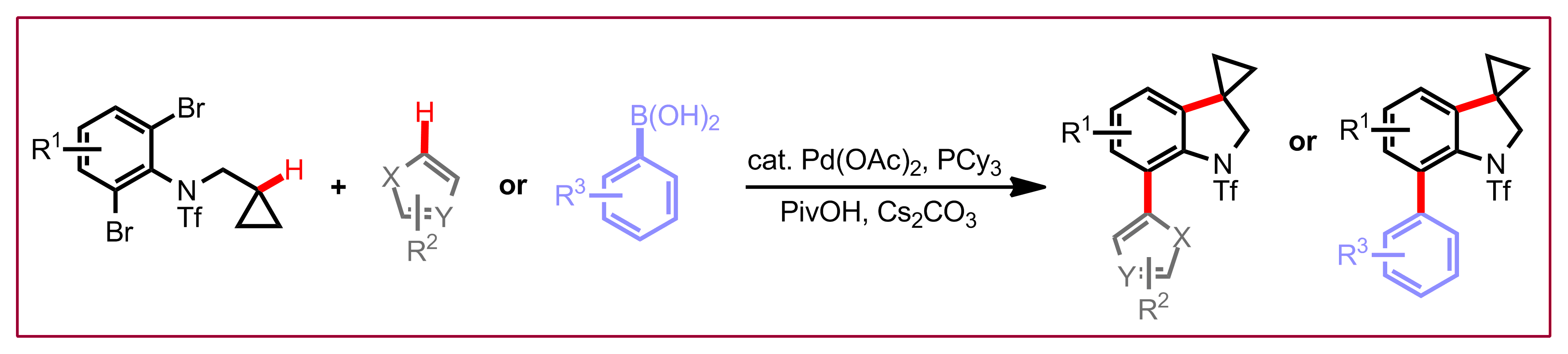
B. Ye, N. Cramer, J. Am. Chem. Soc. 2013, 135, 636-639: “A Tunable Class of Chiral Cp Ligands for Enantioselective Rhodium(III)-Catalyzed C-H Allylations of Benzamides”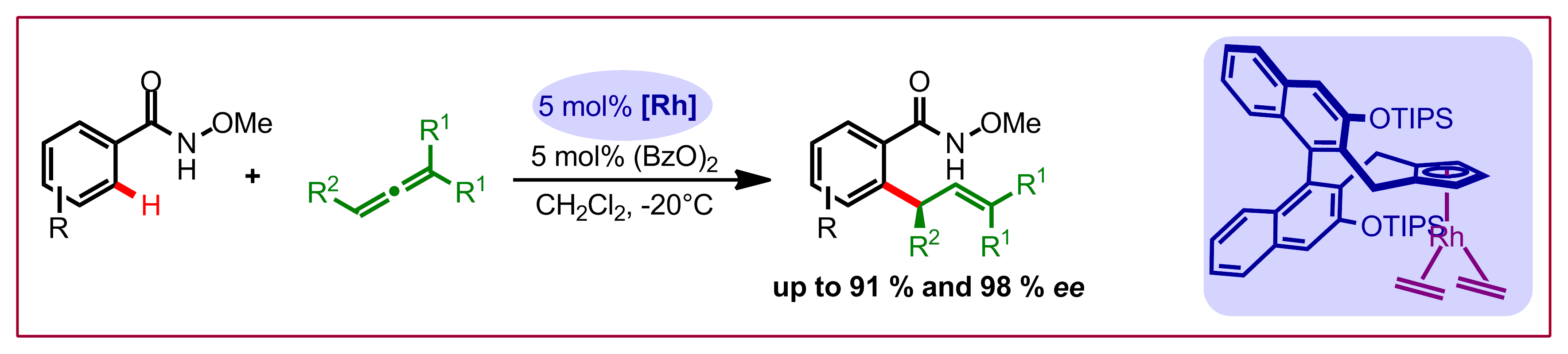
2012
N. Cramer, Chimia 2012, 66, 869-872: “Teaching Enantioselectivity to C-H Bond Functionalizations: Initial Steps of a Rather Long Shot”
T. Saget, N. Cramer, Angew. Chem. Int. Ed. 2012, 51, 12842-12845: “Palladium(0)-Catalyzed Enantioselective C-H Arylation of Cyclopropanes: Efficient Access to Functionalized Tetrahydroquinolines”
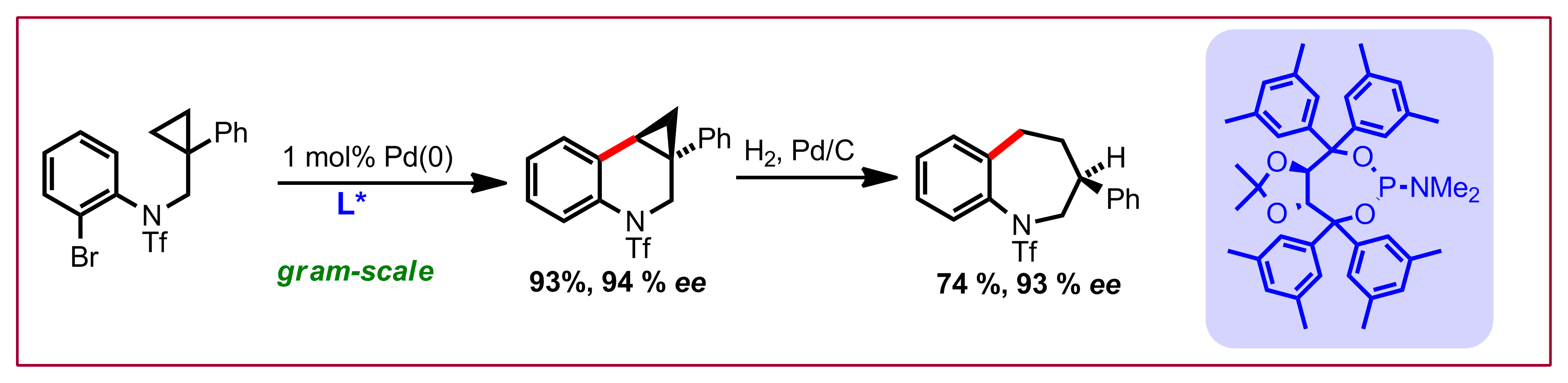
B. Ye, N. Cramer, Science 2012, 338, 504-506: “Chiral Cyclopentadienyl Ligands as Stereocontrolling Element in Asymmetric C-H Functionalization”
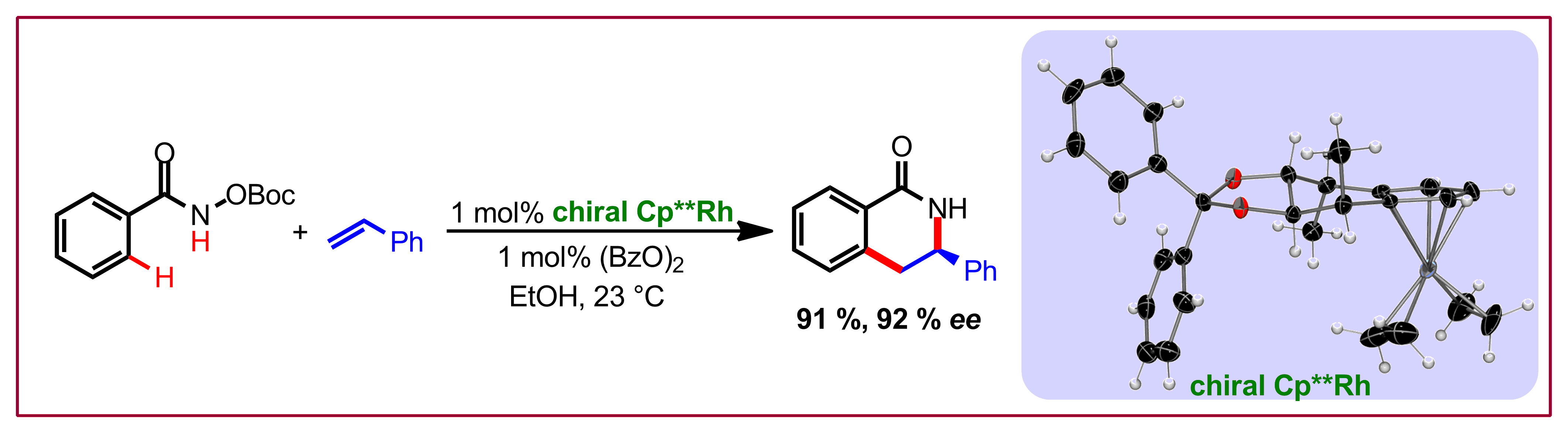
P. A. Donets, T. Saget, N. Cramer, Organometallics 2012, 31, 8040-8046: “Chiral Monodentate Trialkylphosphines Basing on the Phospholane Architecture”
M. Pham, B. Ye, N. Cramer, Angew. Chem. Int. Ed. 2012, 51, 10610-10614: “Access to Sultams by Rhodium(III)-Catalyzed Directed C-H Activations”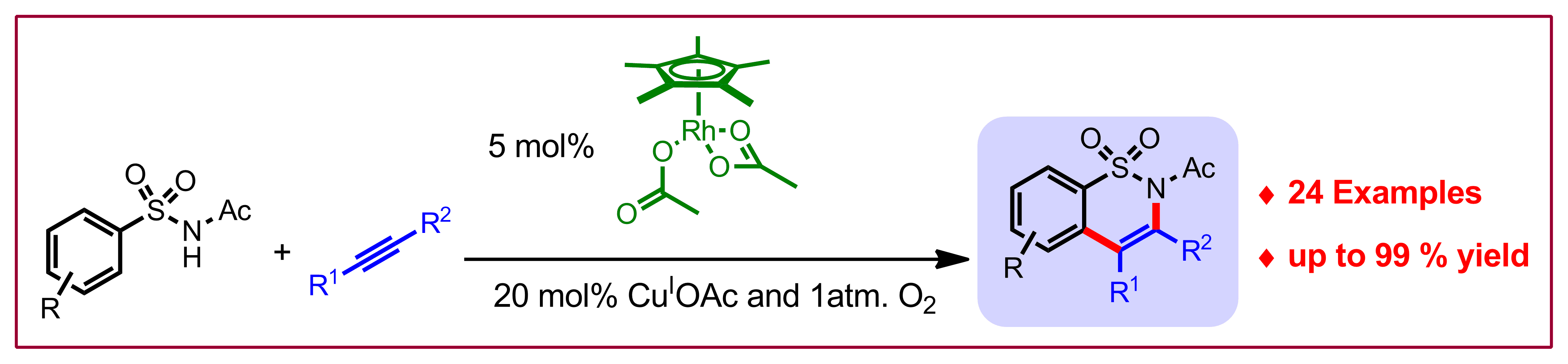
N. Cramer, T. Seiser, “Quaternary Stereogenic Centers by Enantioselective β-Carbon Eliminations from tert-Cyclobutanols” in Asymmetric Synthesis II: More Methods and Applications, (Eds.: M. Christmann and S. Bräse), Wiley-VCH, 2012.
T. Saget, N. Cramer, Chimia 2012, 66, 205-207: “Heteroatom Nucleophile Induced C-C Fragmentations to Access Functionalized Allenes”
T. Saget, S. Lémouzy, N. Cramer, Angew. Chem. Int. Ed. 2012, 51, 2238-2242: “Chiral Monodentate Phosphines and Bulky Carboxylic Acids: Cooperative Effects in Palladium-Catalyzed Enantioselective C(sp3)–H Functionalization”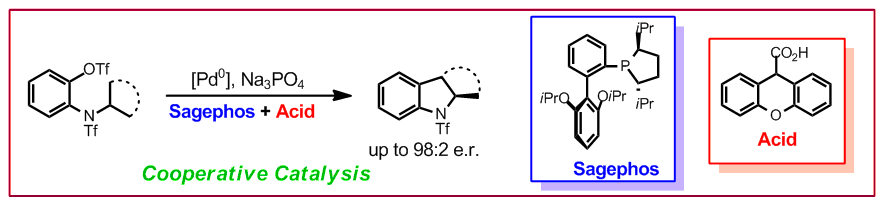
2011
D. N. Tran, N. Cramer Angew. Chem. Int. Ed. 2011, 50, 11098–11102: “Enantioselective Rhodium(I)-Catalyzed [3+2]-Annulations of Aromatic Ketimines Induced by Directed C-H Activations”
B. Winter-Werner, C. Heinis, N. Cramer, Chimia 2011, 65, 818-820 (Conference Report): “The 4th Young Faculty Meeting – Science and Funding in its Different Varieties”
N. Cramer, Chimia 2011, 65, 656-658: “Catalytic Asymmetric Functionalization of Inert Bonds and Synthesis of Bioactive Natural Products”
T. Seiser, T. Saget, D. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2011, 50, 7740–7752: “Cyclobutanes in Catalysis”
T. Saget, N. Cramer, Synthesis 2011, 2369–2371: “Convenient Preparation of Tri-tert-butylphosphonium Tetrafluoroborate”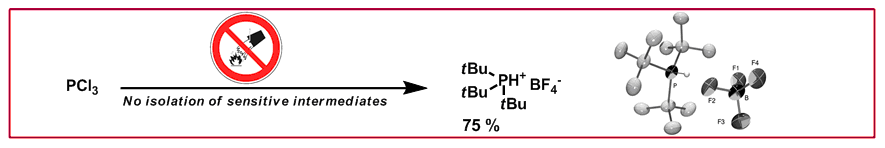
D. N. Tran, N. Cramer, Chimia 2011, 65, 271–273: “Highly Selective Rhodium Catalyzed Domino C-H Activation / Cyclizations”
N. Cramer, T. Seiser, Synlett 2011, 449-460: “β-Carbon Elimination from Cyclobutanols: A Clean Access to Alkylrhodium Intermediates Bearing a Quaternary Stereogenic Center”
M. Waibel, N. Cramer, Chem. Commun. 2011, 345–348: “Desymmetrizations of meso-tert-norbornenols by rhodium(I)-catalyzed enantioselective retro-allylations” 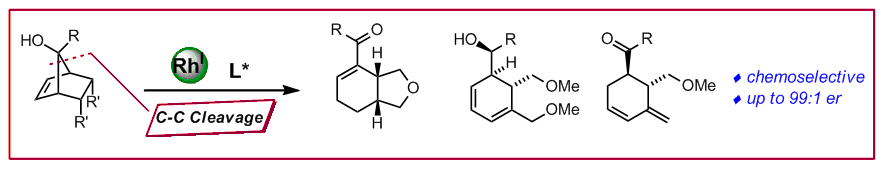
2010
T. Seiser, N. Cramer, Angew. Chem. Int. Ed. 2010, 49, 10163–10167: “Rhodium(I)-catalyzed 1,4-Silicon Shift of Unactivated Silanes from Aryl to Alkyl: Enantioselective Synthesis of Indanol Derivatives”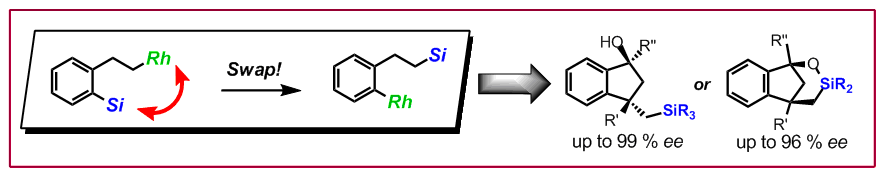
T. Saget, N. Cramer, Angew Chem. Int. Ed. 2010, 49, 8962–8965: “Heteroatom-Nucleophile-Induced C-C Fragmentations: Synthesis of Allenes and Entry to Domino Reactions”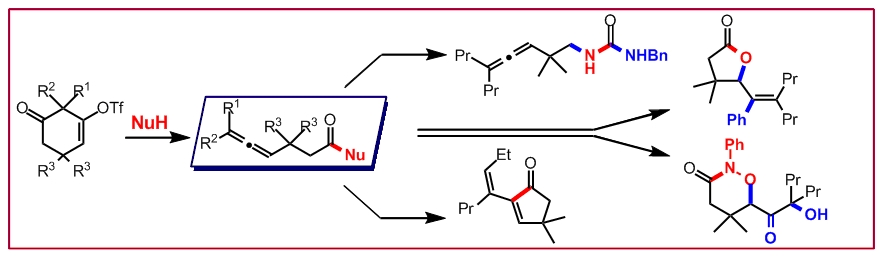
D. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2010, 49, 8181–8184: “Syn-Selective Rhodium(I)-Catalyzed Allylations of Ketimines Proceeding through a Directed C-H Activation/Allene Addition Sequence”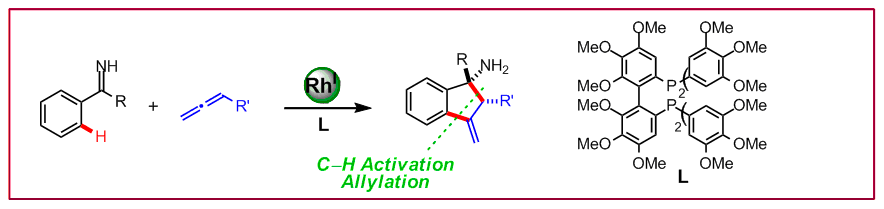
T. Seiser, G. Cathomen, N. Cramer, Synlett 2010, 1699‒1703: “Enantioselective Construction of Indanones from Cyclobutanols Using a Rhodium-Catalyzed C‒C / C‒H / C‒C Bond Activation Process” 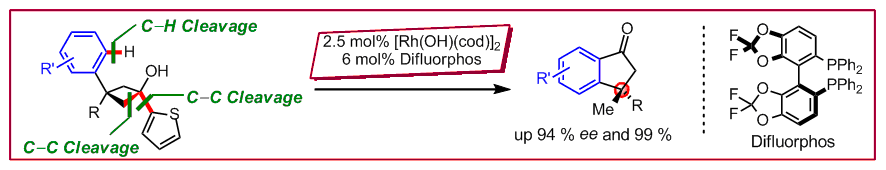
M. Waibel, N. Cramer, Angew. Chem. Int. Ed. 2010, 49, 4455‒4458: “Palladium-Catalyzed Arylative Ring-Opening Reactions of Norbornenols: Entry to Highly Substituted Cyclohexenes, Quinolines, and Tetrahydroquinolines”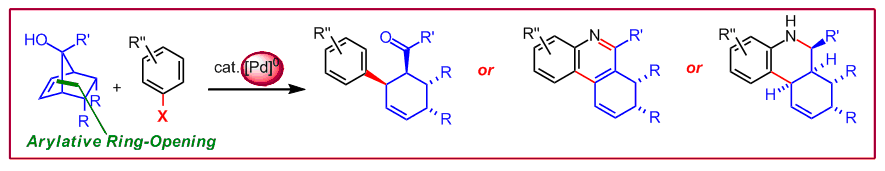
T. Seiser, N. Cramer, Chimia 2010, 64, 153-156: “Enantioselective Rhodium-Catalyzed C-C Bond Activations”
T. Seiser, N. Cramer, J. Am. Chem. Soc. 2010, 132, 5340-5342: “Rhodium-Catalyzed C-C Bond Cleavage: Construction of Acyclic Methyl Substituted Quaternary Stereogenic Centers”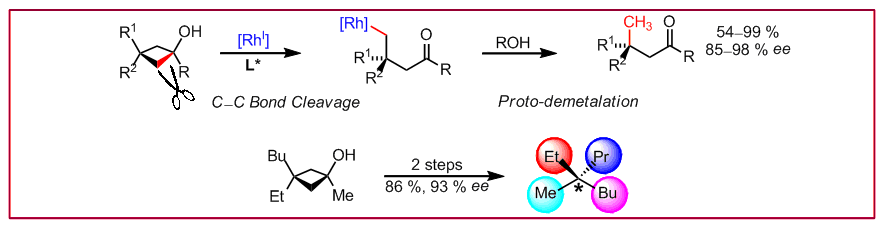
D. T. Ngoc, M. Albicker, L. Schneider, N. Cramer, Org. Biomol Chem. 2010, 8, 1781‒1784: “Enantioselective assembly of the benzo[d]xanthene tetracyclic core of anti-influenza active natural products”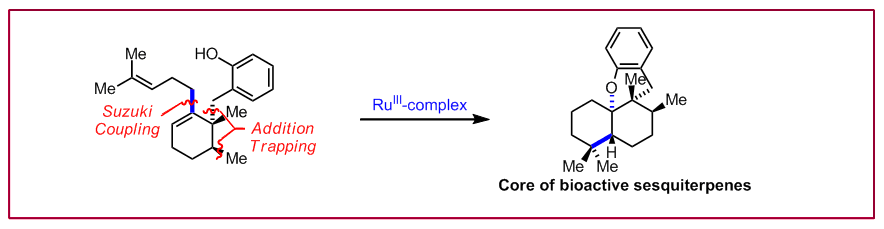
T. Seiser, N. Cramer, Chem. Eur. J. 2010, 16, 3383-3391: “Rhodium(I)-Catalyzed Enantioselective Activation of Cyclobutanols: Formation of Cyclohexane Derivatives with Quaternary Stereogenic Centers”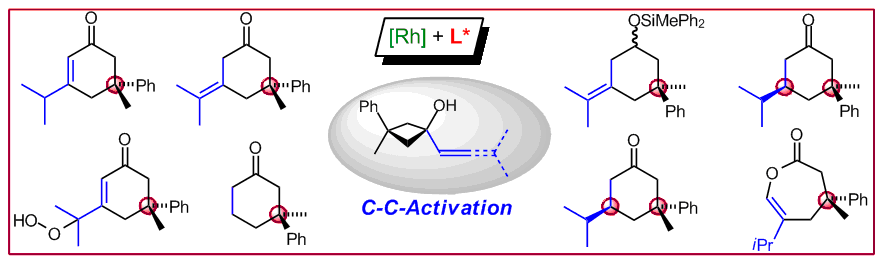
2009
M. R. Albicker, N. Cramer, Angew. Chem. Int. Ed. 2009, 48, 9139-9142: “Enantioselective Palladium-Catalyzed Direct Arylations at Ambient Temperature: Access to Indanes with Quaternary Stereocenters”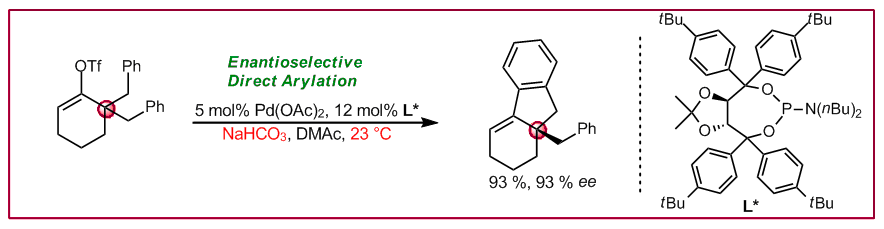
N. Cramer, J. Waser, Chimia 2009, 63, 512-515 (Conference Report): “The 44th EUCHEMS Conference on Stereochemistry (Bürgenstock Conference 2009) Brunnen, May 17–22, 2009”
T. Seiser, O. A. Roth, N. Cramer, Angew. Chem. Int. Ed. 2009, 48, 6320-6323: “Enantioselective Synthesis of Indanols from tert-Cyclobutanols Using a Rhodium-Catalyzed C-C/C-H Activation Sequence”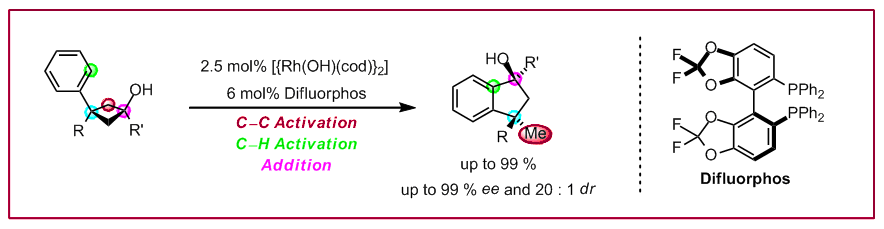
T. Seiser, N. Cramer, Org. Biomol. Chem. 2009, 7, 2835-2840: “Enantioselective metal-catalyzed activation of strained rings” 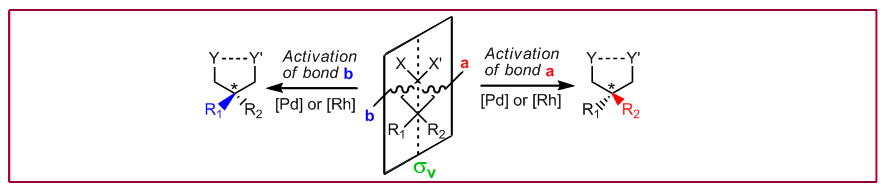
T. Seiser, N. Cramer, Chimia 2009, 63, 19-22: “Syntheses and Biological Activity of the HDAC Class I Inhibitor Largazole”
2008
T. Seiser, N. Cramer, Angew. Chem. Int. Ed. 2008, 47, 9294–9297: “Enantioselective C-C Bond Activation of Allenylcyclobutanes: Access to Cyclohexenones with Quaternary Stereogenic Centers” 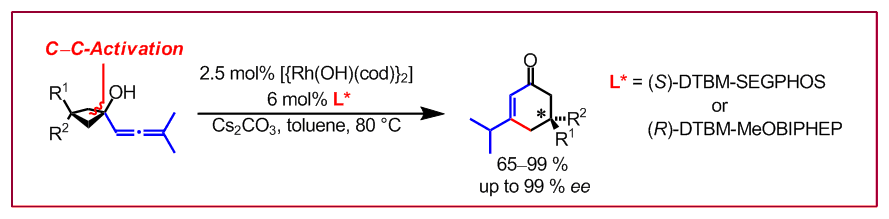
T. Seiser, F. Kamena, N. Cramer, Angew. Chem. Int. Ed. 2008, 47, 6483-6485: “Synthesis and Biological Activity of Largazole and Derivatives”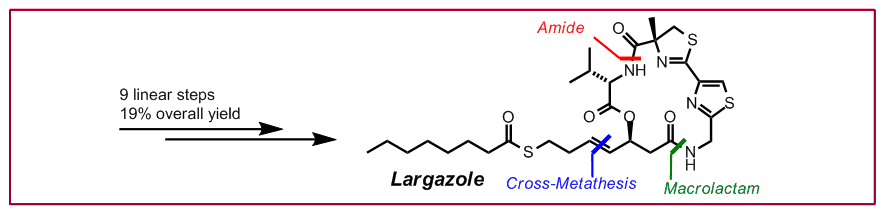
Postdoc and Graduate Work
B. M. Trost, N. Cramer, S. M. Silverman, J. Am. Chem. Soc. 2007, 129, 12396-12397: “Enantioselective Construction of Spirocyclic Oxindolic Cyclopentanes by Palladium-Catalyzed Trimethylenemethane-[3+2]-Cycloaddition”
B. M. Trost, N. Cramer, H. Bernsmann, J. Am. Chem. Soc. 2007, 129, 3086-3087: “Concise Synthesis of (±)-Marcfortine B”
N. Cramer, S. Helbig, A. Baro, S. Laschat, R. Diestel, F. Sasse, D. Mathieu, C. Richter, G. Kummerlöwe, B. Luy, H. Schwalbe, Chem. Bio. Chem. 2008, 9, 2474–2486: “Synthesis and Biological Properties of Cylindramide Derivatives: Evidence for Calcium-Dependent Cytotoxicity of Tetramic Acid Lactams”
S. Helbig, S. Sauer, N. Cramer, S. Laschat, A. Baro, W. Frey, Adv. Synth. Catal. 2007, 349, 2331–2337: “Chiral Bicyclo[3.3.0]octa-2,5-dienes as Steering Ligands in Substrate-Dependent Rhodium-Catalyzed 1,4-Addition of Arylboronic Acids to Enones”
T. Ohashi, N. Cramer, T. Ishimizu, S. Hase, Anal. Biochem. 2006, 352, 182-187: “Preparation of UDP-Galacturonic Acid Using UDP-Sugar Pyrophosphorylase”
N. Cramer, S. Laschat, A. Baro, Organometallics 2006, 25, 2284-2291: “Chiral Phosphites and Phosphoramidites Based on the Tropane Skeleton and Their Application in Catalysis”
N. Cramer, M. Buchweitz, S. Laschat, W. Frey, A. Baro, D. Mathieu, C. Richter, H. Schwalbe Chem. Eur. J. 2006, 12, 2488-2503: “Total Synthesis and NMR Investigations of Cylindramide”
N. Cramer, S. Laschat, A. Baro, H. Schwalbe, C. Richter, Angew. Chem. Int. Ed. 2005, 44, 820-822: “Enantioselective Total Synthesis of Cylindramide”
N. Cramer, J. Juretschke, S. Laschat, A. Baro, W. Frey, Eur. J. Org. Chem. 2004, 1397-1400: “Acid-Promoted Retro-Mannich Reaction of N-Protected Tropenones to 2-Substituted Pyrroles”
N. Cramer, S. Laschat, A. Baro, Synlett 2003, 14, 2178-2181: “Enzymatic Resolution of Tropinone Derivatives”
N. Cramer, S. Laschat, A. Baro, W. Frey, Synlett 2003, 14, 2175-2177: “Enantioselective Desymmetrization of Tropinone Derivatives by Hydroboration”
R. Ducray, N. Cramer, M. A. Ciufolini, Tetrahedron Lett. 2001, 42, 9175-9178: “Homo-Brook Route to Benzazocenols and Congeners via Allylsilane-Derived Aziridines”