Reactor Development (Segmented Flow Tubular Reactor – SFTR)
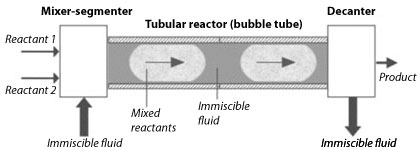
Although many beautiful powders have been prepared and discovered in laboratories at the g or even the mg level, transposing these new powders to the kg production scale is often the stumbling block that condemn these powders to “research discoveries” and does not allow them to create new innovative materials. During the past 15 years, LTP has developed a Segmented Flow Tubular Reactor (SFTR) which allows the production of kg size batches needed for full testing of new nanosized powders. The SFTR overcomes the scale-up problem by segmenting the reactants into micro-reactors in a continuous tubular process.
 |
|
|
|
|
Schematic representation of the Segmented Flow Tubular Reactor (SFTR)
|
|
||
 |
 |
| Pictures of the tubular section | |
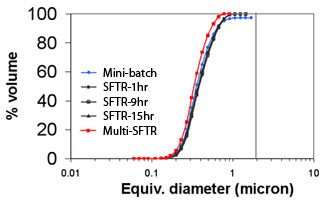
 The process generates identical micro-reactors in which the reagents are thoroughly mixed thus avoiding heterogeneous reaction conditions encountered in classical large batch reactors. As a result, particle size distribution width is distinctly narrower and phase purity and morphology control are significantly improved. Production of calcium carbonate (calcite) was carried out during a 25 hour continuous run and the powder characteristics (dv50 = 0.39 µm, span = 1.06) were maintained throughout the whole run.
The process generates identical micro-reactors in which the reagents are thoroughly mixed thus avoiding heterogeneous reaction conditions encountered in classical large batch reactors. As a result, particle size distribution width is distinctly narrower and phase purity and morphology control are significantly improved. Production of calcium carbonate (calcite) was carried out during a 25 hour continuous run and the powder characteristics (dv50 = 0.39 µm, span = 1.06) were maintained throughout the whole run.
 |
 |
|
a)
|
b)
|
|
a) Micrograph and b) particle size distribution of calcium carbonate (calcite) precipitated using the Segmented Flow Tubular Reactor technology during a 15-hour continuous run. Particle size distributions measured after 1, 9 and 15 hours are nearly identical to the particle size distribution of the powder produced in a 20 ml batch reactor.
|
|
This process allows the precipitation of homogeneous products with narrow particle size distributions, enhanced control of particle morphology, polymorph selectivity and better stoechiometry control; all these features have been demonstrated for precipitates as varied as CaCO3, BaTiO3, Mn(1-x)NixC2O4·2H2O, YBa oxalates, copper oxalate, ZnS, ZnO, iron oxides, TiO2. The SFTR is also ideally suited to producing nanosized powders.
Publications:
-
Solid State Ionics, 171(1-2), 135-140, (2004).
-
AIChE Journal, 46(6), 1241 – 1252, (2000).
-
Chemical Engineering & Technology, 26(3), 303 – 305, (2003).
-
Aimable, T. Strachowski, E. Wolska, W. Lojkowski, P. Bowen, Comparison of two innovative precipitation systems for ZnO and Al-doped ZnO nanoparticle synthesis, Processing and Application of Ceramics 4 [3] (2010) 107–114.
-
Aimable, M.T. Buscaglia, V. Buscaglia, P. Bowen, Polymer-assisted precipitation of ZnO nanoparticles with narrow particle size distribution, J.Eur. Ceram.Soc. 30(2) (2010), 591-598.
-
Aimable N. Jongen, A. Testino, M. Donnet, J. Lemaitre, H. Hofmann and P. Bowen, “Precipitation of nanosized and nanostructured powders: process intensification using SFTR”, applied to BaTiO3, CaCO3 and ZnO – Chem. Eng. & Techn., 34(3) 344-352 (2011).