A. Lund, G. V. Manohara, A.-Y. Song, K. M. Jablonka, C. P. Ireland, L. A. Cheah, B. Smit, S. Garcia, and J. A. Reimer, Characterization of Chemisorbed Species and Active Adsorption Sites in Mg–Al Mixed Metal Oxides for High-Temperature CO2 Capture Chem. Mater. (2022) doi:n10.1021/acs.chemmater.1c03101
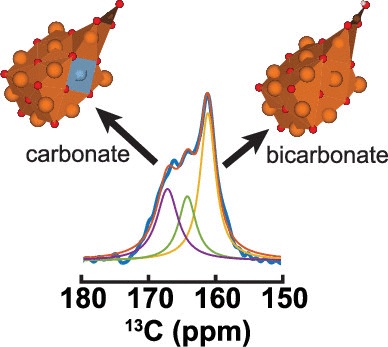
Abstract: Mg–Al mixed metal oxides (MMOs), derived from the decomposition of layered double hydroxides (LDHs), have been purposed as adsorbents for CO2 capture of industrial plant emissions. To aid in the design and optimization of these materials for CO2 capture at 200 °C, we have used a combination of solid-state nuclear magnetic resonance (ssNMR) and density functional theory (DFT) to characterize the CO2 gas sorption products and determine the various sorption sites in Mg–Al MMOs. A comparison of the DFT cluster calculations with the observed 13C chemical shifts of the chemisorbed products indicates that mono- and bidentate carbonates are formed at the Mg–O sites with adjacent Al substitution of an Mg atom, while the bicarbonates are formed at Mg–OH sites without adjacent Al substitution. Quantitative 13C NMR shows an increase in the relative amount of strongly basic sites, where the monodentate carbonate product is formed, with increasing Al/Mg molar ratios in the MMOs. This detailed understanding of the various basic Mg–O sites presented in MMOs and the formation of the carbonate, bidentate carbonate, and bicarbonate chemisorbed species yields new insights into the mechanism of CO2 adsorption at 200 °C, which can further aid in the design and capture capacity optimization of the materials.