B. Á. Novotny, S. Majumdar, A. Ortega-Guerrero, K. M. Jablonka, E. Moubarak, N. Gasilova, N. P. Domingues, R.-A. Kessler, E. Oveisi, F. M. Ebrahim, and B. Smit, Nonuniform Chiralization of Metal–Organic Frameworks Using Imine Chemistry ACS Mater. Au (2025) doi: 10.1021/acsmaterialsau.4c00139
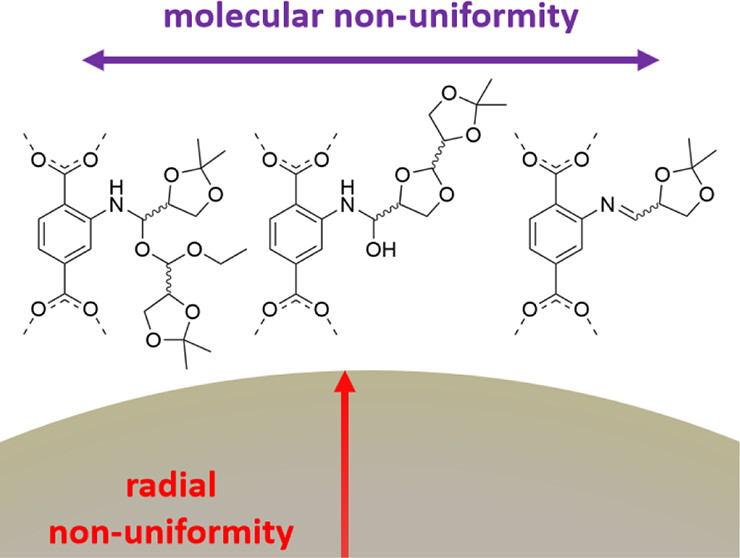
Abstract: Homochiral metal–organic frameworks (MOFs) are exceptional media for heterogeneous enantiodifferentiation processes. Modifying available achiral structure-bearing MOF scaffolds is a preferred method to extend this class of materials. Reported postsynthetic covalent chiralizations generally lead to uniform, site-specific modifications. The use of chemically versatile modifying agents, like aldehydes, may instead result in the statistical formation of chemically nonuniform anchored products. In addition, the use of such modifying agents gives rise to spatial nonuniformities in the radial direction, due to prohibited diffusion through the MOF bulk. The advantageous grain structure formation plus molecular nonuniformity greatly increase the complexity of such systems. The use of such modifying agents, therefore, necessitates a broader holistic characterization. The present work explores the adaptation of imine chemistry for postsynthetic chiralization. A chiral aldehyde and a chiral ketone are probed on two amine-functionalized MOF substrates─MIL-125 NH2 and UiO-66 NH2. The UiO-66 NH2 modified with the natural product-derived (R)-2,2-dimethyl-1,3-dioxolane-4-carboxaldehyde ((R)-1 aldehyde) is found to have the best performance in terms of reactivity and MOF stability. A comprehensive toolbox of methods was demonstrated to robustly characterize the obtained material. This includes high-resolution accurate mass electrospray ionization mass spectrometry (HRAM-ESI-MS) to reveal the competing reactions that yield a set of oligomer-rich structures. In silico modeling correctly predicts the localization of the modification. The modification is found to be covalent and chiral and mainly proceeds through imine formation, resulting in a surface enantioselector display formation. Restricted diffusion lengths in the solid phase infer good retention of resolving power in ascending van Deemter régimes in chromatography. Meeting this criterion makes the yielding material a promising potential stationary phase candidate for performant chromatographic enantioseparations.